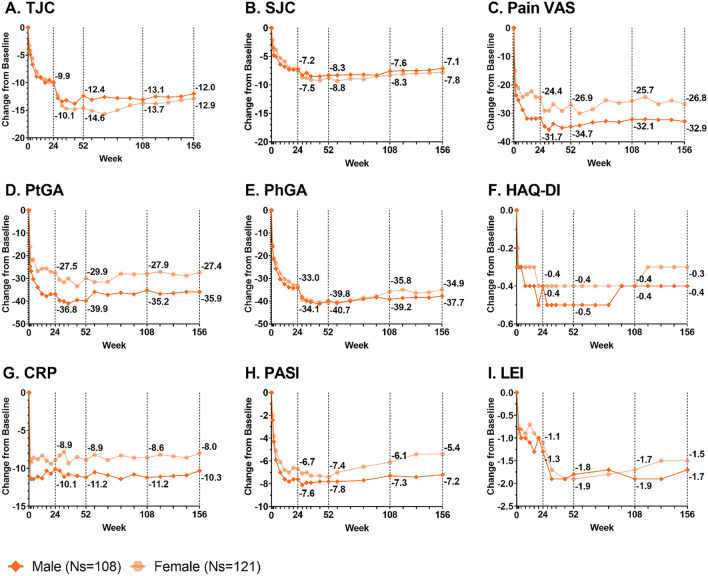
Fig. 3.
Changes from baseline in male and female patients treated with ixekizumab Q4W for TJC (a), SJC (b), Pain VAS (c), PtGA (d), PhGA (e), HAQ-DI (f), CRP (g), PASI (h), and LEI (i) through 156 weeks. Missing data were imputed by mBOCF. TJC includes 68 joints; SJC includes 66 joints. Pain VAS, PtGA, and PhGA are measured on a 0–100 scale. HAQ-DI is measured on a 0–3 scale. CRP is measured in mg/L. PASI is measured on a 0–72 scale. LEI is measured on a 0–6 scale. Change from baseline in PASI was measured in 77 male patients and 64 female patients with ≥ 3% body surface area affected at baseline. Change from baseline in LEI was measured in 57 male patients and 79 female patients with LEI > 0 at baseline. CRP C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, LEI Leeds Enthesitis Index, mBOCF modified baseline observation carried forward, Ns number of patients in subgroup, Pain VAS Pain Visual Analog Scale, PASI Psoriasis Area and Severity Index, PhGA Physician’s Global Assessment of Disease Activity, PtGA Patient’s Global Assessment of Disease Activity, SJC swollen joint count, TJC tender joint count, Q4W every 4 weeks