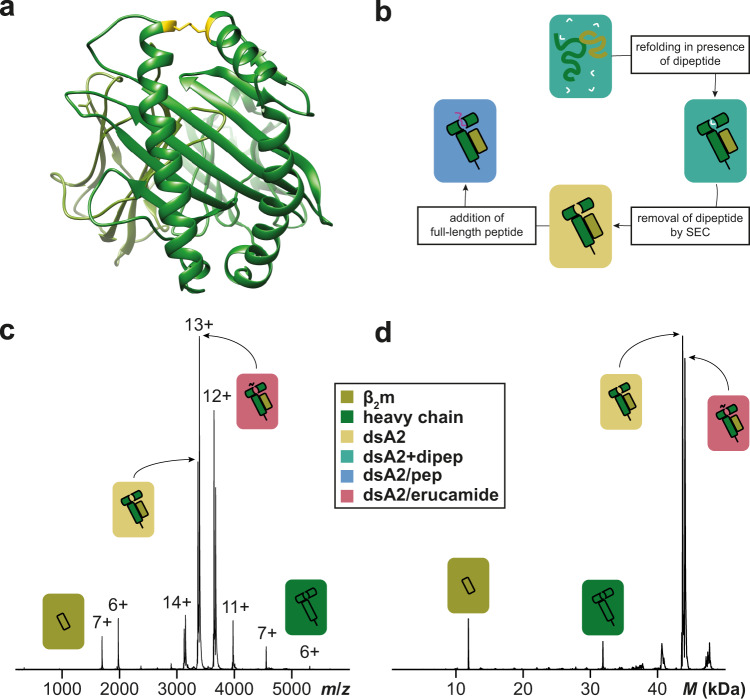
Fig. 1. Disulfide-stabilized HLA-A*02:01.
a Crystal structure of empty dsA2 (PDB 6TDR14,68) seen as a top view of the peptide pocket. β2m is shown in olive and the heavy chain in green. The stabilizing disulfide bond (positions 84 and 139 mutated to cysteines) is depicted in yellow. b dsA2 is refolded in presence of GM or GL dipeptide, which is then removed via size-exclusion chromatography. Afterward, full-length peptides can bind into the empty binding groove. Raw (c) and deconvoluted (d) native mass spectra of peptide-free dsA2 were recorded at an acceleration voltage of 25 V. The empty dsA2 is the predominant species (yellow). In addition, dsA2/erucamide (coral) and dissociated β2m (olive) and heavy chain (green) can be seen.