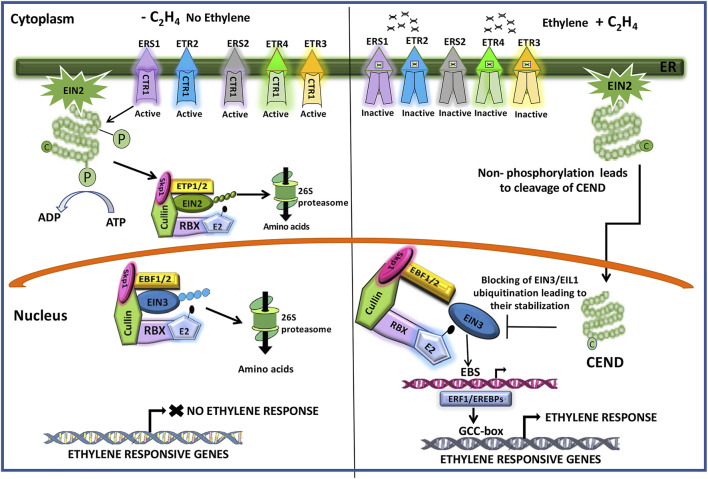
FIGURE 8.
Cartoon showing the molecular mechanism of ethylene action. The histidine kinase domain of CTR1 (constitutive triplet response1) of the ethylene receptors (ERS1, ERS2, ETR2, ETR3, ETR4) keeps on phosphorylating the CEND (C-terminal end) of the membrane bond protein EIN2 (ethylene insensitive2). This follows its recognition by the F-box proteins ETP1/2 (EIN2 targeting protein1/2) for ubiquitination by the SCF (Skp1-Culin-F-box) E3 ligase complex that additionally contains RBX, a RING box protein, and the E2 ubiquitin-conjugating enzyme and degradation of the ubiquitinated protein by the 26S proteosome system. Upon binding of ethylene to the receptors, the CTR1 kinase activity is inhibited, and the CEND gets detached and moves to the nucleus where it blocks the ubiquitination of EIN3 (ethylene insensitive3)/EIL1 (ethylene insensitive3 like1), preventing its 26S proteosomal degradation. Accumulation of EIN3 promotes it to bind to the EBS (Ein3/EIL1-binding site) in the promoter of the ethylene responsive factor1 (ERF1) and other ethylene-responsive element binding proteins (EREBPs) to drive their expression. ERF1/EREBPs in turns bind to the GCC-box in the promoter of the ethylene-responsive genes, leading to ethylene response. In the absence of EIN2 in the nucleus, EIN3 is recognized by the F-Box proteins EBF1/2 (EIN3-binding F-Box Protein1/2) for ubiquitination through the SCF (Skp1-Culin-F-box) E3 ligase complex, followed by its degradation by the 26S proteosome. Adapted from Ji and Guo (2013).