Abstract
Purpose
To review the sustained effect of COVID-19 on rhegmatogenous retinal detachment (RRD) baseline characteristics and outcomes.
Methods
This was a retrospective consecutive case series at the Birmingham and Midlands Eye Centre including patients undergoing primary RRD repair between 23 March and 31 December 2017–2019 (Group 1) and 2020 (Group 2). The deciles of indices of multiple deprivation (IMD) were determined by postcode to group patients into least deprived (IMD1-5) and most deprived (IMD6-10).
Results
In total we reviewed 1310 patients, 1003 in Group 1 and 307 in Group 2. Relative to 2017–2019, during the first lockdown, we observed (a) a reduction in the number of patients with RRD, (b) an increase in macula-off detachments, (c) an increase in RRD primary failure, and (d) that the least deprived had proportionately higher primary failure than the most deprived (p = 0.049) with a higher detachment rate than the pre-COVID-19 period (p = 0.010) and increased presentations of macula-off detachment. During the second lockdown, these differences were not observed.
Conclusion
The previously observed findings of lower presentation rates of RRD during the beginning of the first lockdown and the decreased number of macula-on RRD were not sustained over a longer period of observation or found to recur after a second national lockdown. Patients from areas with the least socioeconomic deprivation seemed to be more negatively affected by the first lockdown, with later presentation and higher rates of re-detachments compared with the most deprived during the first lockdown. Our findings offer reassurance that patient behaviour and health services had adapted to the pandemic by the second national lockdown.
Supplementary Information
The online version of this article (10.1007/s00717-022-00521-0) contains supplementary material, which is available to authorized users.
Keywords: Retinal detachment, COVID-19, Eye, Vitreoretinal, Retinopexy, Retina
Abstract
Ziel
Ziel der vorliegenden Arbeit war es, anhaltende Auswirkungen von COVID-19 auf Ausgangsmerkmale und Ergebnisse rhegmatogener Netzhautablösungen (RRD) zu untersuchen.
Methoden
Es handelt sich um eine retrospektive Serie aufeinanderfolgender Fälle im Birmingham and Midlands Eye Centre mit Patienten, bei denen eine primäre RRD-Therapie zwischen 23. März und 31. Dezember der Jahre 2017–2019 (Gruppe 1) und des Jahres 2020 (Gruppe 2) erfolgt war. Die Zehntelsegmente (Dezile) der Indizes der Mehrfachbenachteiligung (IMD) wurden anhand der Postleitzahlen bestimmt, mit denen in am wenigsten benachteiligte (IMD1-5) und am meisten benachteiligte Patienten (IMD6-10) eingeteilt wurde.
Ergebnisse
Daten von 1310 Patienten wurden ausgewertet, 1003 in Gruppe 1 und 307 in Gruppe 2. Im Vergleich zu 2017–2019 stellte sich während des ersten Lockdowns a) eine geringere Anzahl von Patienten mit RRD heraus, b) ein Anstieg bei Makulaablösungen, c) ein Anstieg des Versagens der Primärtherapie bei RRD und d), dass die am wenigsten Benachteiligten ein proportional höheres Versagen der Primärtherapie als die am meisten Benachteiligten aufwiesen (p = 0,049) – bei einer höheren Ablösungsrate als in der Prä-COVID-19-Phase (p = 0,010) und mehr Fällen mit Makulaablösung. Während des zweiten Lockdowns wurden diese Unterschiede nicht beobachtet.
Schlussfolgerung
Die frühere Beobachtung niedrigerer Vorstellungsraten von RDD-Patienten während des anfänglichen ersten Lockdowns und die verminderte Anzahl von RRD ohne Makulaablösung bestand nicht anhaltend über einen längeren Beobachtungszeitraum oder trat nicht erneut nach einem zweiten nationalen Lockdown auf. Patienten aus Gebieten mit der geringsten sozioökonomischen Benachteiligung schienen stärker negativ vom ersten Lockdown betroffen zu sein – aufgrund späterer ärztlicher Vorstellung und höherer Raten erneuter Ablösungen – als die am stärksten Benachteiligten. Die vorliegenden Ergebnisse sprechen dafür, dass das Patientenverhalten und die Einrichtungen zur medizinischen Versorgung sich beim zweiten nationalen Lockdown an die Pandemie angepasst hatten.
Schlüsselwörter: Netzhautablösung, COVID-19, Auge, Vitreoretinal, Retinopexie, Retina
Introduction
The 2019 novel coronavirus (COVID-19) has created an international public health disaster that has resulted in the widespread disruption of healthcare services worldwide [1]. The World Health Organization (WHO) declared COVID-19 to be a global pandemic on 11 March 2020 [2]. In the United Kingdom, routine face-to-face outpatient care was put on hold from 23 March 2020 following the announcement that a national lockdown would be in force from 16 March 2020. Subsequently, a significant decrease in attendances to ophthalmic emergency departments was observed [3], including at our tertiary referral centre, the Birmingham and Midland Eye Centre, where we observed a 25–30% decrease in attendances to our ophthalmic emergency department.
The impact of COVID-19 is not solely on the provision of health services, but it has also radically altered the behaviours of patients regarding when to seek medical help for fear of contracting the virus. Worldwide, there are changes seen in the clinical presentations of vitreoretinal (VR) pathology with delays in seeking treatment for retinal detachments thereby resulting in greater proportions of primary proliferative vitreoretinopathy (PVR; [3–7]). We have also observed that the number of patients undergoing primary retinopexy during the first lockdown period in England, prior to measures being relaxed, halved and that the rhegmatogenous retinal detachment (RRD) rate has also increased following primary retinopexy treatment over a similar period of time compared to the previous year [8].
Currently, most published reports are focused on the change in the timing and clinical presentation of RRD during the first few months of lockdown [4–6]. Therefore, we sought to investigate the sustained effected of COVID-19 and the impact of repeated and persistent lockdowns that occurred in the UK on the presentation of primary RRD to our tertiary VR service. As the impact of socioeconomic deprivation has been determined to be ever more divisive in healthcare outcomes [9] during the lockdown [10], this study also investigated whether this was a factor affecting clinical outcomes. The significant periods covered in this study include the first national lockdown from 23 March to 4 July 2020, the additional local restrictions from 15 September in Birmingham and the second national lockdown from 5 November 2020 until the remainder of the year of 2020.
Methods
We present a single-centre, retrospective, continuous comparative study to analyse all patients who underwent primary retinal detachments at the Birmingham and Midland Eye Centre (BMEC). Our study period was from the start of full lockdown on 23 March to 31December for four consecutive years (2017 to 2020). Group 1 corresponds to the 3 years pre-COVID-19 primary detachments (23 March to 31 December 2017–2019) and Group 2 is designated to during-COVID-19 primary detachments (23 March to 31 December 2020). As the third national lockdown initiated on 6 January 2021 in the UK, December 2020 represented the last full month without a national lockdown. For calculating the retinal re-detachment rate, patients whose postcode was outside our catchment area were excluded as these patients may have had further surgery at the referring unit and only patients who had primary retinal detachments until September were analysed (to allow time to capture the 3 months of retinal re-detachment rate). Due to the complex variation in restrictions that took place during this time period, we have presented data in our figures divided by months to capture the trends that occurred due to the sustained effect of COVID-19. All the data were extracted from electronic patient records (EPR, Medisoft Ophthalmology, Medisoft Limited, Leeds, UK).
Our primary outcome measures were the characteristics and outcomes of RRD before and during the pandemic. Primary RRDs repaired by PPV were selected to risk adjust for case complexity and enable more meaningful comparison between groups. Retinal re-detachment rates were based on repeat retinal detachment surgery in the same eye within 3 months. All patients who had prior VR surgery were excluded. All RRD surgery were performed with either (a) transconjunctival 23-gauge pars plana vitrectomy (PPV) with cryotherapy and/or endolaser retinopexy and gas or oil tamponade, (b) scleral buckle and cryotherapy, or a combined buckle with PPV.
Deprivation ranking
The English Government developed the English Indices of Deprivation 2019 (IoD), which are a measure of relative deprivation based on seven different domains of deprivation. These are combined to output a weighted relative measure of deprivation, the Index of Multiple Deprivation (IMD)—weighting in brackets (%): [11] Income Deprivation (22.5%), Employment Deprivation (22.5%), Education, Skills and Training Deprivation (13.5%), Health Deprivation and Disability (13.5%), Crime (9.3%), Barriers to Housing and Services (9.3%) and Living Environment Deprivation (9.3%). The IMD is given a rank by the postcode area in England and ranks are converted to deciles, 1 indicating the highest level of deprivation and 10 being the most affluent. The full postcode of patients was used to extract the IMD decile and correlated with retinal detachment rate by month across Group 1 and Group 2. The IMD decile was dichotomised for analysis: (a) most deprived: deciles 1–5 (IMD-A); and (b) least deprived: deciles 6–10 (IMD-B).
The following data were collated: age of patient, gender, presence of high myopia (defined as greater than six dioptres of myopia), preoperative and postoperative visual acuity (VA), surgery type, tamponade used (if applicable), date of surgery and IMD decile rank.
Statistical analysis
Statistical significance was defined as p < 0.05. Prior to analysis, the normality of continuous variables was assessed using the Shapiro–Wilk test and was found not to be normally distributed. Hence, data are primarily reported as medians and interquartile ranges (IQRs) throughout. The Mann–Whitney U test was used to compare two independent groups (age and VA). The Wilcoxon signed rank test was used for two-paired VA data. Fisher’s exact test and the chi-squared test were used for nominal variables. Bonferroni correction was applied for multiple statistical analysis. Scores for VA, corresponding to count fingers (CF), hand movements (HM), perception of light (PL) and no PL (NPL), were substituted with 2.10, 2.40, 2.70 and 3.00 LogMAR, respectively, using a conversion tool developed by Moussa et al. [12], in keeping with previous publications from the national ophthalmology database group [13]. All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 28.0 (IBM Corp., Armonk, NY, USA).
Results
A summary of the clinical characteristics of primary RRD according to Group 1 and Group 2 is presented in Table 1. A total of 1310 primary RRD characteristic outcomes were analysed. In Group 1, primary RRD surgery was performed on 1003 patients (or 334 per year) and in Group 2 this was performed on 307 patients in the same study period.
Table 1.
Baseline clinical characteristics of primary retinal detachment patients determined by COVID-19 status
| Total | Pre-COVID-19 Group 1 |
During-COVID-19 Group 2 |
p | |
|---|---|---|---|---|
| Total | 1310 | 1003 | 307 | |
| Age (years) | 58 (50 to 67) | 58 (49 to 67) | 59 (50 to 69) | 0.150 |
| Gender (% male) | 831 (63.4%) | 639 (63.7%) | 192 (62.5%) | 0.735 |
| Deprivation rankb | ||||
| Most deprived (IMD-A) | 712 (55.2%) | 546 (55.0%) | 166 (55.7%) | 0.894 |
| Least deprived (IMD-B) | 578 (44.8%) | 446 (45.0%) | 132 (44.3%) | |
| Laterality (% right) | 696 (53.1%) | 536 (53.4%) | 160 (52.1%) | 0.695 |
| High myopia (% yes) | 95 (7.3%) | 69 (6.9%) | 26 (8.5%) | 0.378 |
| RD characteristic | ||||
| Macula-on | 630 (50.2%) | 469 (49.2%) | 161 (53.3%) | 0.235 |
| Trauma related | 14 (1.1%) | 10 (1.0%) | 4 (1.3%) | 0.750 |
| Any PVR | 106 (8.1%) | 82 (8.2%) | 24 (7.8%) | 0.905 |
| PVR C | 55 (4.2%) | 44 (4.4%) | 11 (3.6%) | 0.627 |
| Surgery type | ||||
| PPV | 1141 (87.1%) | 866 (86.3%) | 275 (89.6%) | 0.146 |
| Scleral buckle | 142 (10.8%) | 114 (11.4%) | 28 (9.1%) | 0.295 |
| Combined | 27 (2.1%) | 23 (2.3%) | 4 (1.3%) | 0.363 |
| Tamponade choicea | ||||
| SF6 | 338 (28.9%) | 286 (32.2%) | 52 (18.6%) | < 0.001 |
| C2F6 | 346 (29.6%) | 246 (27.7%) | 100 (35.8%) | 0.008 |
| C3F8 | 262 (22.4%) | 183 (20.6%) | 79 (28.3%) | 0.007 |
| Silicone oil | 148 (12.7%) | 123 (13.8%) | 25 (9.0%) | 0.050 |
| Heavy silicone oil | 59 (5.1%) | 38 (4.3%) | 21 (7.5%) | 0.028 |
| Pre-op VA (LogMAR) | 0.48 (0.18 to 1.48) | 0.48 (0.18 to 1.78) | 0.48 (0.18 to 1.48) | 0.625 |
Age and VA are reported as median (interquartile range). The Mann–Whitney U test was used to compare continuous variables between groups. Fisher’s exact test was used to compare nominal groups
Statistical significance in bold
RD retinal detachment, VA visual acuity, PVR proliferative vitreoretinopathy
aPPV cases only, unknown tamponade in 15 patients (13 and 2 from each group, respectively)
bIndices of multiple deprivation not available in 20 patients
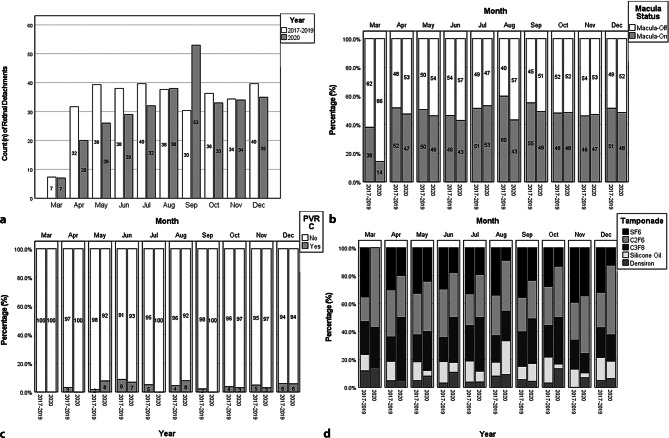
No statically significant difference was detected between the two groups in all types of PVR, high myopia status, gender, trauma-related retinal detachments or type of surgery performed (buckle or PPV). However, shorter-acting tamponade sulphur hexafluoride (SF6) was used less in Group 2 (p < 0.001) and longer-acting tamponades were used more in Group 2 (C2F6: p = 0.008, C3F8: p = 0.007, [heavy] silicone oil: p = 0.028). The different characteristics of primary RRD by each month across both groups are presented in Fig. 1; Fig. 1a shows the relatively lower RRD number in Group 2 that steadily increases and spikes considerably in September 2020. This aligns with the lifting of the first national lockdown on 4 July and the introduction of further local restrictions mid-September 2020 in Birmingham. Macula-on and macula-off retinal detachments for each month were compared between the two groups and the results are represented in Fig. 1b. There is a consistently higher proportion of macula-off RRDs in Group 2, from the first lockdown until the lifting of the lockdown in July where the trend temporarily reverses. Little difference in PVR C levels was seen between groups across the months in our study period. There remains a persistently low utilisation of short-acting SF6 tamponade in Group 2 relative to Group 1 across all months (Fig. 1d). Supplementary Fig. 1 demonstrates the trend in gas tamponade use, reiterating the shift toward longer-acting gas tamponade and, overall, less oil (Silicone oil + Heavy silicone oil) tamponade.
Fig. 1.
Monthly breakdown of pre-COVID-19 and during-COVID-19 and retinal detachment characteristics and outcomes. First lockdown: 23 March to 4 July. Additional local restrictions in Birmingham from 15 September. Second national lockdown: 5 November. Significant restrictions until end of data collection period. a Mean monthly (23 March to 31 December) count of retinal detachments (2017 to 2019) compared to 2020. b Proportions of macula-off and macula-on retinal detachments by period. c Proportions of PVR C retinal detachments by period. d Proportions of tamponade agent choice by period
A summary of clinical outcomes of primary retinal detachment surgery according to Group 1 and Group 2 is found in Table 2. In this analysis, 1044 patients were included, and 266 patients excluded as not enough time had elapsed for the 3‑month retinal re-detachment rate to be calculated and for patients outside our catchment area.
Table 2.
Outcome of primary retinal detachment patients determined by COVID-19 status
| Totala | Pre-COVID-19 Group 1 |
During-COVID-19 Group 2 |
p | |
|---|---|---|---|---|
| Total | 1044 | 859 | 185 | – |
| Time between surgery and VA (days) | 134 (62 to 336) | 175 (71 to 372) | 70 (34 to 105) | < 0.001 |
| 3‑Month detachment rate (% yes) | 141 (13.5%) | 112 (13.0%) | 29 (15.7%) | 0.344 |
| Days to detachment surgery | 44 (27 to 72) | 47 (28 to 81) | 35 (23 to 62) | 0.156 |
| Pre-Op VA (LogMAR) | 0.48 (0.18 to 1.78) | 0.48 (0.18 to 1.78) | 0.60 (0.18 to 2.10) | 0.168 |
| Post-Op VA (LogMAR) | 0.30 (0.18 to 0.78) | 0.30 (0.18 to 0.78) | 0.48 (0.18 to 1.00) | 0.006 |
| LogMAR gain | 0.12 (−0.12 to 0.60) | 0.12 (−0.12 to 0.60) | 0.18 (−0.12 to 0.70) | 0.820 |
Continuous data are reported as median (interquartile range). The Mann–Whitney U test was used to compare continuous variables between groups. The chi-squared (> 2 groups) and Fisher’s exact test (2 groups) were otherwise used to compare nominal groups
Statistical significance in bold
a1044 patients included (266 patients excluded as not enough time elapsed for re-detachment rate to be calculated and for patients outside our catchment area)
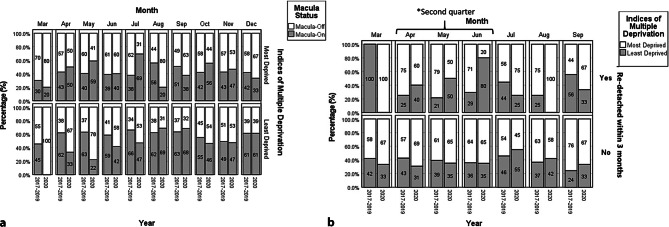
We report an overall RRD rate of 141 (13.5%) eyes following primary retinopexy in our cohort. There was an increase from 112 (13.0%) eyes in Group 1 to 29 (15.7%) eyes in Group 2 (p = 0.344). The retinal re-detachment rate by month between the two groups is shown in Fig. 2. Although initially the retinal re-detachment rate was higher In Group 2, this normalised towards the lifting of the second lockdown by June 2020. If we factor in socioeconomic deprivation as demonstrated in Fig. 3a, we find that the least deprived group had a higher proportion of macula-off RRD in 2020 until August, relative to the pre-COVID-19 years. In Fig. 3b, the least deprived group were significantly more affected by lockdown regulations than the most deprived group. In the second quarter (April to June), the least deprived group had a higher re-detachment rate during-COVID-19 than pre-COVID-19 (p = 0.010), which was not found in the most deprived group (p = 1.000). Of the patients that experienced re-detachment, significantly more of them were from the least deprived group in the second quarter during-COVID-19 (p = 0.049) where no difference was detected pre-COVID-19 (p = 0.525). Similarly, pre-COVID-19, the most deprived had significantly worse post-operative visual acuity than the least deprived (Supplementary Fig. 2, p < 0.001). However, during COVID-19, no difference was detected (p = 0.402).
Fig. 2.
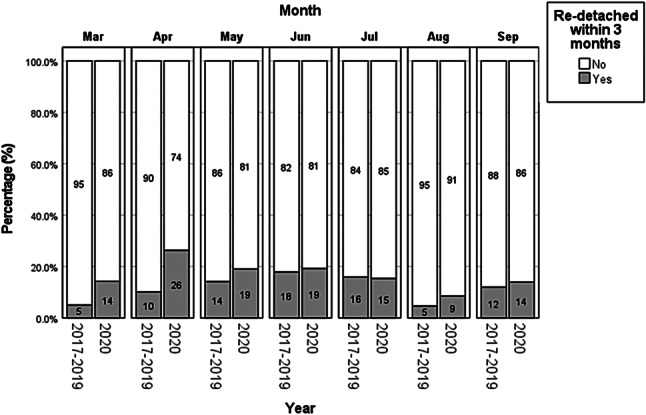
Monthly breakdown of pre-COVID-19 and during-COVID-19 and re-detachment rate. Retinal detachment rate by month (3-month detachment rate available until September 2020) comparing pre-COVID-19 and during-COVID-19 groups
Fig. 3.
Deprivation status and monthly breakdown of pre-COVID-19 and during-COVID-19 groups and retinal detachment characteristics and outcomes by deprivation status. a Patients from areas of least socioeconomic deprivation had a higher proportion of macula-off RRD in 2020 relative to the pre-COVID-19 years, an observation not made with the most deprived group. b The effect of deprivation status and re-detachment rate before and during the COVID-19 pandemic. The least deprived group were significantly more affected by lockdown regulations than the most deprived group. In the second quarter (April to June), the least deprived group had a higher re-detachment rate during-COVID-19 then pre-COVID-19 (p = 0.010), which was not found in the most deprived group (p = 1.000). Asterisk Of the patients who experienced re-detachment, significantly more of them were from the least deprived group in the second quarter during-COVID-19 (p = 0.049) where no difference was detected pre-COVID-19 (p = 0.525)
No significant difference was found in pre-operative visual acuities (p = 0.168) between both groups. Group 2 reported significantly worse post-operative VAs (p = 0.006). However, no difference between groups was found in LogMAR gain (p = 0.820).
Discussion
Our study looked at the sustained effect of the COVID-19 pandemic and the resulting lockdowns and restrictions on RRD clinical characteristics and outcomes compared to pre-pandemic presentations. Lower numbers of presentations with RRD were initially observed during the first lockdown, consistent with findings from other studies [3–7]. Numbers of RRD presentations increased steadily, approaching Group 1 levels by the lifting of the first national lockdown in July 2020 (with a considerable surplus in September 2020 relative to Group 1 noted). The higher proportion of macula-off RRD during the period of the first lockdown, suggestive of late presentation to ophthalmic services, is also consistent with the findings of other studies [3–7]. However, we found these trends were observed only for the first lockdown period and further restrictions in September and the second national lockdown did not see a difference with pre-COVID-19 levels. A common trend across all our subgroups is that after the introduction of the second national lockdown, little difference is seen between both groups and healthcare systems, and patient attitudes to presentation seem to have reverted to pre-COVID-19 levels.
During the first lockdown period, Poyser et al. observed higher rates of males presenting to their ophthalmic service with VR pathologies [5], and our previous study also observed the same trend with regards to patients presenting with retinal tears requiring primary retinopexy [8]. This gender disparity was not demonstrated in this study with similar proportions of each gender in both groups.
From the introduction of the second national lockdown period (October 2020, with local restrictions from September 2020), presentations to the ophthalmic service, a surrogate marker for patient attitudes, suggest that the numbers have returned to those of pre-COVID-19 levels. The reasons for this are likely multi-factorial. Relatively little was known about COVID-19 at the time of the first lockdown being enforced, including which groups of people were most at risk. By the timing of the introduction of the second lockdown, patients who may have waited for the resolution of the pandemic prior to presenting may have not anticipated the length of time the restrictions would remain in place. The decline in presentation to emergency services or primary care services was also demonstrated across a range of medical conditions (e.g. myocardial infarctions, cerebrovascular accidents) at the beginning of the lockdown period [14, 15]. However, patient attitudes are unlikely to be the only reason for the reduced presentation. Access to primary care services was also likely to be a contributing factor. Primary care contact for key physical and mental health conditions dropped substantially after lockdown restrictions were introduced in March 2020 and though it showed signs of recovery later on, by July 2020 (lifting of the first national lockdown) it remained below that of pre-lockdown levels [16]. NHS 111, the 24/7 urgent telephone helpline, saw a sharp increase in its utilisation in March 2020 in response to patients being unable to contact primary care contacts; however, over 50% of the calls were not answered. This may have also contributed to the reduction in timely presentations at the start of the first lockdown period as patients may have been unable to obtain their triage advice [17]. With the on-going pandemic, health services had a chance to adapt their policies and emergency services, with more understanding regarding the condition (including how to reduce transmission), to be more accessible to the most at-risk patients. As such, the findings seen at the start of the first lockdown have not been sustained and the numbers presenting to our service have recovered to pre-pandemic levels.
Increase in PVR at presentation, again a marker of delayed presentation, has been repeatedly reported. Patel et al. reported an increase in PVR at presentation in their cohort (13.4% vs. 4.5% in the control group, p = 0.03; [7]) as did Awad et al. (24.3% vs 9.8% in the control group, p = 0.0471; [4]). This correlated with their findings of decreased macula-on RRD presentations compared to their pre-pandemic control groups. This was concerning as PVR is the leading cause of retinal detachment surgery failure and studies suggested that over time, we may be faced with the prospect of more complex and challenging retinal detachments to manage with poorer prognoses [4, 7, 18, 19]. Our study, which investigated a larger cohort of patients over a longer period of time, did not observe this finding. Rates of any PVR at presentation were similar in both Groups 1 and 2 (8.2% and 7.8%, respectively) with a slight decrease in PVR‑C noted in Group 2 over the study period (however, this did not reach statistical significance). Our current cohort of patients in this study also includes a significantly greater number of patients than the previously mentioned studies [6]. It is unclear why our cohort of patients did not experience a significantly higher rate of PVR compared to other studies. One reason may be the maintenance of our dedicated ophthalmic theatres for emergency work in our tertiary referral unit during this period. Other units which share theatres with other specialities may have seen a delay in treatment due to the overall disruption of services, particularly around the time of the first lockdown. Throughout the study period, however, our service was able to maintain dedicated VR theatres for emergency work and hence this would have contributed to minimising any delays to treatment. We also found differences in gas tamponade relative to the pre-Covid-19 years. When comparing the year 2020 with each year since 2017 (Supplementary Fig. 1), the year 2020 represents a reduction in oil tamponade from the increasing trend in the years preceding and an increase in longer-acting gas tamponade. Since oil tamponade requires removal of oil at a later date, which takes theatre capacity, there was a shift towards longer-acting gas tamponade use in an attempt to avoid multiple procedures.
We have previously reported that socioeconomic deprivation leads to higher failure rates in a risk-adjusted cohort [20]. However, this study was also the first to look at the impact of socioeconomic deprivation on RRD rates as affected by the pandemic. In our cohort, those from areas of higher socioeconomic deprivation were not as adversely impacted as those from areas of least socioeconomic deprivation. Patients from areas of least socioeconomic deprivation had a higher proportion of macula-off RRD in 2020 relative to the pre-COVID-19 years, an observation not made with the most deprived group (Fig. 3a). Additionally, pre-COVID-19, the least deprived had significantly better visual outcomes compared to during the COVID-19 pandemic.
This observation is in stark contrast to the disparity in the number of cases and mortality rates from COVID-19 seen between those living in areas with the highest levels of socioeconomic deprivation versus those in the lowest. Those living in the areas with the highest levels of socioeconomic deprivation saw significantly higher number of cases of COVID-19 and more than double the mortality rates compared to areas with lower levels of deprivation [21]. This could suggest that those in areas of higher deprivation are less likely to modify their behaviour to comply with lockdown regulations (and hence would be at higher risk of infection) but as a result of not modifying their behaviour, they are more likely to attend health services sooner than those from areas of least deprivation. This is an interesting observation and would benefit from further studies.
Study limitations and strengths
The limitations of our study include its retrospective nature and the lack of case randomisation. Additionally, the lens status at the time of final VA measurements was not available. A prospective study would not have been possible for this comparison because of the serious health effects of COVID-19 on society and the healthcare logistics behind the planning of a prospective study. Besides, we have used RRD surgery as a rate of failed retinal detachment surgery at 3 months, which is a relatively short time to assess the retinal re-detachment rate following primary RRD repair. However, in our study, a significant difference was detected in this period for the least deprived group. Nevertheless, our study is the largest at assessing the sustained effect of COVID-19 with multiple lockdowns on clinical presentation and post-RRD repair outcomes in our group of patients as well as the differences between different levels of IMD.
Conclusion
The previously observed findings of lower presentation rates of retinal detachments during the beginning of the first lockdown and the decreased number of macula-on RRD were not shown to be sustained over a longer period of observation or found to recur after a second national lockdown was enforced in the UK. Presenting numbers and characteristics of retinal detachments were similar in our study to those observed pre-pandemic. Increased rates of PVR at presentation were also not observed in our study compared to pre-pandemic levels. Socioeconomic deprivation was found to be a factor in patients presenting to our service, with those from areas of least socioeconomic deprivation presenting later and with increased rates of re-detachments compared to those from areas of higher socioeconomic deprivation. Overall, this study contributes to the literature on the impact of COVID-19 on the wider healthcare service and its potential implications for the future. Our findings are, however, reassuring that patient behaviour and health services have adapted to the pandemic and by the second national lockdown, the characteristics of presentation of retinal detachments had normalised to pre-COVID-19 levels.
Supplementary Information
Supplementary Fig. 1: Tamponade choice across 4 years. The year 2020 represents a reduction in oil tamponade from the increasing trend in the years preceding and an increase in longer-acting gas tamponade
Supplementary Fig. 2: Visual outcome by deprivation index before and during COVID-19. In the years 2017–2019, the most deprived had significantly worse post-operative visual acuity than the least deprived (p < 0.001). However, during COVID-19, no difference was detected (p = 0.402). Mann–Whitney U test for all p values.
Acknowledgments
Funding
The authors received no funding for the production of this manuscript.
Author Contribution
All authors have made substantial contributions to the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Declarations
Conflict of interest
G. Moussa, M.O. Qadir, S.W. Ch’ng, K.S. Lett, A. Mitra, A.K. Tyagi, A. Sharma and W. Andreatta declare, that they have no competing interests.
Ethical standards
This study was registered and approved by our local clinical effectiveness team (Clinical Effectiveness Department, Sandwell General Hospital: reference number: 1593). As this was a retrospective anonymized study, as per our local protocol from our Clinical Effectiveness Department, and as per national guidelines from the National Code of Clinical Research, and the Health Research Authority (HRA), this study has ethical approval exemption and no patient consent was required for participation. All procedures were completed prior to the design of this study. Patients were diagnosed and treated according to local guidelines and agreements and written consent from patients was acquired prior to all procedures as clinically indicated. This study does not report on the use of new or experimental protocols.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Madabhavi I, Sarkar M, Kadakol N. CoviD-19: a review. Monaldi Arch Chest Dis. 2020;p:248–258. doi: 10.4081/monaldi.2020.1298. [DOI] [PubMed] [Google Scholar]
- 2.OMS. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19. Accessed 20 May 2020, p. 4..
- 3.Wickham L, Hay G, Hamilton R, Wooding J, Tossounis H, da Cruz L, et al. The impact of COVID policies on acute ophthalmology services—experiences from Moorfields Eye Hospital NHS Foundation. Trust. Eye. 2020;34:1189–1192. doi: 10.1038/s41433-020-0957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad M, Poostchi A, Orr G, Kumudhan D, Zaman A, Wilde C. Delayed presentation and increased prevalence of proliferative vitreoretinopathy for primary rhegmatogenous retinal detachments presenting during the COVID-19 pandemic lockdown. Eye. 2021;35:1282–1283. doi: 10.1038/s41433-020-1056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poyser A, Deol SS, Osman L, Sivagnanasithiyar T, Kuht HJ, Manrique R, et al. Impact of COVID-19 pandemic and lockdown on retinal detachments. Eye. 2021;35:2322–2323. doi: 10.1038/s41433-020-01137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arjmand P, Murtaza F, Eshtiaghi A, Popovic MM, Kertes PJ, Eng KT. Impact of the COVID-19 pandemic on characteristics of retinal detachments: the Canadian experience. Canadian Journal of Ophthalmology [Internet]. Elsevier B.V.; 2021 [cited 2021 Apr 9];56:88–95. [DOI] [PMC free article] [PubMed]
- 7.Patel LG, Peck T, Starr MR, Ammar MJ, Khan MA, Yonekawa Y, et al. Clinical presentation of rhegmatogenous retinal detachment during the COVID-19 pandemic: a historical cohort study. Ophthalmology. 2021;128:686–692. doi: 10.1016/j.ophtha.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moussa G, Samia-Aly E, Andreatta W, Lett KS, Mitra A, Sharma A, et al. The impact of COVID-19 on primary retinopexy in preventing retinal detachment in a tertiary eye hospital emergency department. European Journal of Ophthalmology. SAGE Publications Ltd; 2022 [cited 2021 Apr 19];32:534–8 [DOI] [PubMed]
- 9.Moussa G, Hodson J, Gooch N, Virdee J, Penaloza C, Kigozi J, et al. Calculating the economic burden of presumed microbial keratitis admissions at a tertiary referral centre in the UK. Eye (Lond); 2021 [cited 2021 Jul 16];35:2146–54. [DOI] [PMC free article] [PubMed]
- 10.Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. Journal of Epidemiology and Community Health [Internet]. BMJ Publishing Group; 2020 [cited 2021 Apr 19];74:jech-2020-214401. [DOI] [PMC free article] [PubMed]
- 11.Ministry of Housing Communities and Local Government. English indices of deprivation 2019. National Statistics. 2019. http://imd-by-postcode.opendatacommunities.org/imd/2019. Accessed 02.08.2020.
- 12.Moussa G, Bassilious K, Mathews N. A novel excel sheet conversion tool from Snellen fraction to LogMAR including ‘counting fingers’, ‘hand movement’, ‘light perception’ and ‘no light perception’ and focused review of literature of low visual acuity reference values. Acta Ophthalmol. 2021 doi: 10.1111/aos.14659. [DOI] [PubMed] [Google Scholar]
- 13.Day AC, Donachie PHJ, Sparrow JM, Johnston RL. The royal college of ophthalmologists’ national ophthalmology database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–560. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams R, Jenkins DA, Ashcroft DM, Brown B, Campbell S, Carr MJ, et al. Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: a retrospective cohort study. Lancet Public Health. 2020;5:e543–e550. doi: 10.1016/S2468-2667(20)30201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansfield KE, Mathur R, Tazare J, Henderson AD, Mulick AR, Carreira H, et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Heal. 2021;3:e217–e230. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vestesson E, Gardner T. How has NHS 111 shaped public demand for the NHS in England during the pandemic? The Health Foundation; 2020. [Google Scholar]
- 18.Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol. 2004;137:1105–1115. doi: 10.1016/j.ajo.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Shams F, El-Abiary M, Goudie C, Yorston D. Effects of lockdown on retinal detachment incidence in Scotland. Eye [Internet]. Springer Nature; 2021 [cited 2021 Apr 19];35:1279–80. [DOI] [PMC free article] [PubMed]
- 20.Moussa G, Kalogeropoulos D, Ch’ng SW, Lett KS, Mitra A, Tyagi AK, et al. Effect of deprivation and ethnicity on primary macula-on retinal detachment repair success rate and clinical outcomes: a study of 568 patients. PLoS ONE. 2021;16:e0259714. doi: 10.1371/journal.pone.0259714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PHE . Disparities in the risk and outcomes of COVID-19. PHE Publications; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Tamponade choice across 4 years. The year 2020 represents a reduction in oil tamponade from the increasing trend in the years preceding and an increase in longer-acting gas tamponade
Supplementary Fig. 2: Visual outcome by deprivation index before and during COVID-19. In the years 2017–2019, the most deprived had significantly worse post-operative visual acuity than the least deprived (p < 0.001). However, during COVID-19, no difference was detected (p = 0.402). Mann–Whitney U test for all p values.