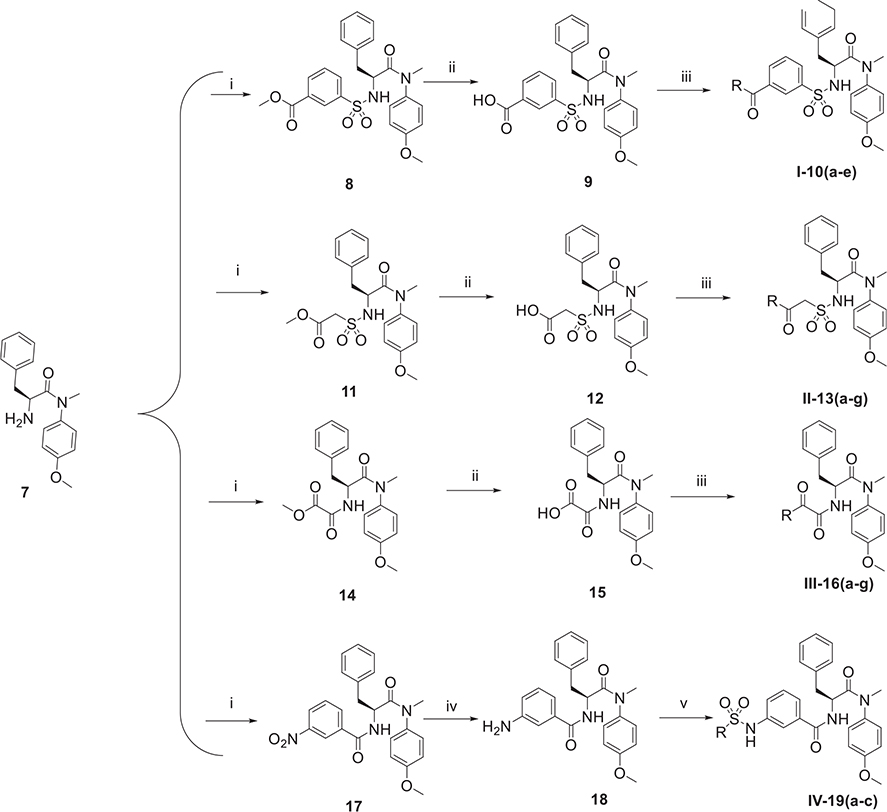
Scheme 2.
The synthetic route of phenylalanine derivatives (series I-IV). Reagents and Conditions: (i) methyl 3-chlorosulfonylbenzoate or methyl 2-(chlorosulfonyl) acetate or methyl chlorooxoacetate or 3-nitrobenzoyl chloride or amine fragments, TEA, CH2Cl2, 0°C, 4 h; (ii) NaOH, THF/H2O (V:V = 1:1), r.t., 2 h; (iii) amine fragments, HATU, DIEA, CH2Cl2, r.t., 8 h; (iv) SnCl2·2H2O, EtOH, N2, r.t., 8 h.(v) sulfonyl chloride substituents, TEA, CH2Cl2, 0 °C, 4 h.