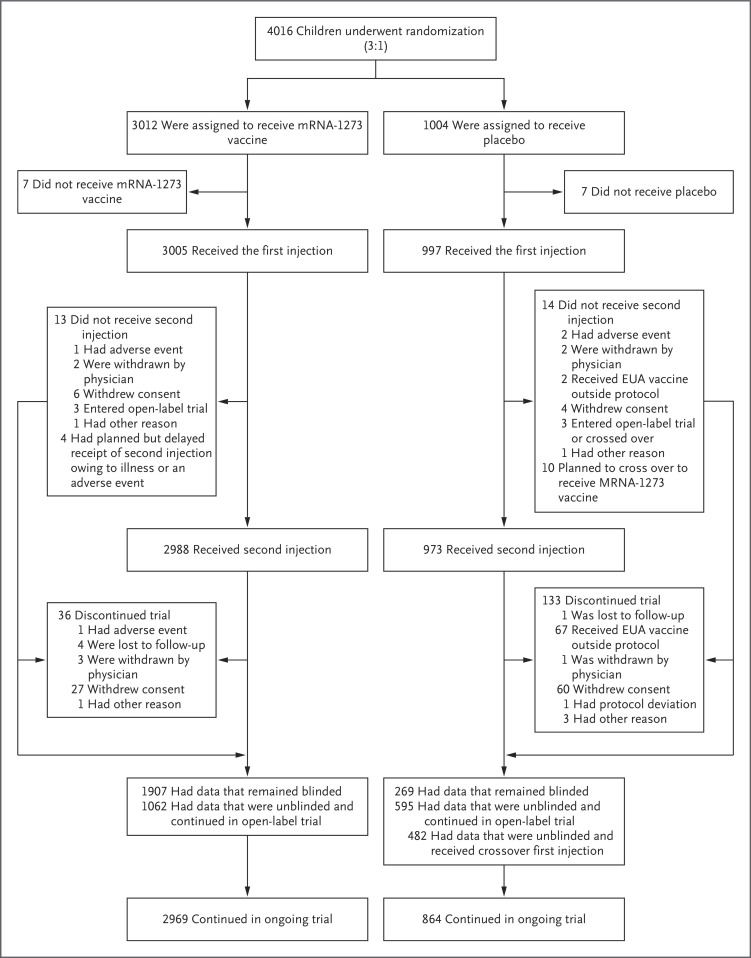
Figure 1. Randomization and Analysis Populations in Part 2 of the Trial.
The populations of trial participants (6 to 11 years of age) who received the mRNA-1273 vaccine at a dose level of 50 μg or placebo are shown. The reasons for not receiving a first injection included withdrawal of consent (in 8 participants), screening failure because of error in randomization (in 5), and physician decision owing to a medication change 1 month before consent (in 1). Two participants who were randomly assigned to receive placebo received the mRNA-1273 vaccine. In the placebo group, the two adverse events were related to coronavirus disease 2019 (Covid-19). In the mRNA-1273 vaccine group, of the 36 participants who discontinued the trial, 9 had received a first injection and 27 had received a second injection. In the placebo group, of the 133 participants who discontinued the trial, 10 had received a first injection and 123 had received a second injection. The number of trial discontinuations includes 9 participants in the vaccine group and 67 participants in the placebo group who had data that were unblinded and discontinued the trial. After October 29, 2021, the date of emergency use authorization (EUA) of the BNT162b2 vaccine for children 5 to 11 years of age, participants became eligible to have their data unblinded. The cutoff date for blinded data was November 10, 2021.