Figure 2.
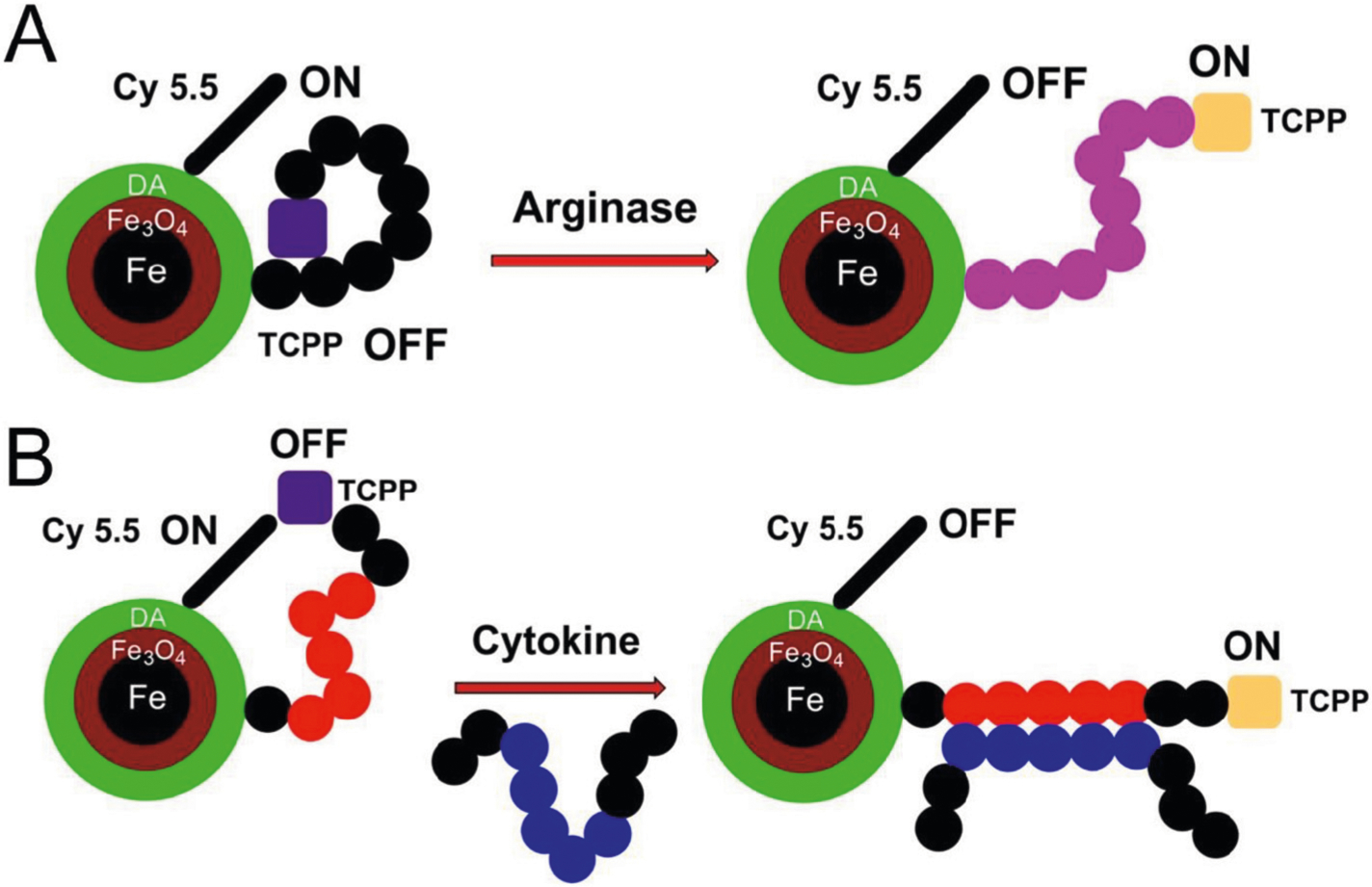
(A) Design principles of the improved nanobiosensor for arginase detection. Due to the presence of seven L-arginine residues in G(R7) TCPP is quenched by means of three quenching mechanisms: 1) plasmonic quenching, 2) FRET, and 3) proton transfer quenching. When L-arginase is modified by arginase (I + II) to L-ornithine, the dynamics of the tether changes, thus reducing plasmonic quenching and FRET. Due to the hydrolytic release of urea from L-arginine, proton transfer quenching is no longer possible, and the reaction ceases. The overall effect is switching on TCPP fluorescence, in response to arginase activity, which can then be quantified. (B) Design principles of nanobiosensors for cytokine/chemokine detection. Instead of a consensus sequence, the tether features a peptide-binding site for an epitope on the cytokine/chemokine of interest. Cytokine/chemokine binding triggers a conformational change of the tether, resulting in a statistical increase of TCPP and Fe/Fe3O4/Cyanine (Cy) 5.5, which decreases both plasmonic quenching and FRET. Consequently, fluorescence increase of TCPP is observed. Whereas the distance dependence of FRET processes decreases with r−6, surface energy transfer, also known as plasmonic quenching,42 drops off according to r−4 characteristics.
