Abstract
We investigated the anticancer effect of the aptamer‐conjugated gemcitabine‐loaded atelocollagen patch in a pancreatic cancer patient–derived xenograft (PDX) model to propose a future potential adjuvant surgical strategy during curative pancreatic resection for pancreatic cancer. A pancreatic cancer PDX model was established. Animals were grouped randomly into a no‐treatment control group; treatment group treated with intraperitoneal gemcitabine injection (IP‐GEM) or aptamer‐conjugated gemcitabine (APT:GEM); and transplant with three kinds of patches: atelocollagen‐aptamer‐gemcitabine (patch I), atelocollagen‐inactive aptamer‐gemcitabine (patch II), and atelocollagen‐gemcitabine (patch III). Tumor volumes and response were evaluated based on histological analysis by H&E staining and Immunohistochemistry (IHC) was performed. Anticancer therapy–related toxicity was evaluated by hematologic findings. The patch I group showed the most significant reduction of tumor growth rate, compared with the no‐treatment group (p < 0.05). However, other treatment groups were not found to show significant reduction in tumor growth rate (0.05 < p < 0.1). There was no microscopic evidence suggesting potential toxicity, such as inflammation, nor necrotic changes in liver, lung, kidney, and spleen tissue. In addition, no leukopenia, anemia, or neutropenia was observed in the patch I group. This implantable aptamer‐drug conjugate system is thought to be a new surgical strategy to augment the oncologic significance of margin‐negative resection in treating pancreatic cancer in near future.
Keywords: anticancer drug, pancreatic cancer, patient‐derived tumor xenograft mouse model, PDTX, PDX
We investigated the anticancer effect of the aptamer‐conjugated gemcitabine‐loaded atelocollagen patch in a pancreatic cancer patient–derived xenograft (PDX) model to propose a new adjuvant strategy during curative surgery. This implantable drug delivery system will be a new surgical strategy to augment oncologic significance of margin‐negative resection in treating pancreatic cancer in near future.
![]()
Abbreviations
- CBC
complete blood cell count
- H&E
hematoxylin and eosin
- PLT
platelet count
- RBC
red blood cell
- WBC
white blood cell
1. INTRODUCTION
Pancreatic cancer is known to be one of the most lethal malignant diseases arising in the gastrointestinal tract. Among other malignant diseases, no improvement of survival outcome of pancreatic cancer has been reported. It is estimated that pancreatic cancer is projected to surpass breast, prostate, and colorectal cancers to become the second leading cause of cancer‐related death by 2030. Therefore, active investigation for developing a new effective strategy to improve the oncologic outcome of pancreatic cancer is urgently needed. Margin‐negative pancreatic resection is known to be the most effective monotherapy for pancreatic cancer; however, cancer recurrences are the rule, and the long‐term survival of resected pancreatic cancer remains around 20%‐30%.
Recently, potent chemotherapeutic agents have been introduced for treating pancreatic cancer, showing outstanding oncologic outcomes; however, the severe adverse effects of systemic chemotherapy prevent all pancreatic cancer patients from taking great advantages of these armaments in clinical oncology. In addition, postoperative local anatomic changes are associated with disconnection of vascularity and may attenuate treatment efficacy of postoperative adjuvant systemic chemotherapy due to poor drug delivery to potential residual cancer cells.
We hypothesized that microscopic residual cancer cells around the surgical bed would be a nidus for future local and systemic recurrences after curative resection of pancreatic cancer, and they could be effectively treated with localized application of an efficient dose of anticancer agent. Therefore, to improve the treatment efficacy of chemotherapeutic agents for microscopic residual pancreatic cancer cells, we have developed a pancreatic cancer–specific aptamer‐conjugated chemotherapeutic agent–loaded collagen patch (Figure 1A), which is supposed to continuously deliver a localized and efficient anticancer.
FIGURE 1.
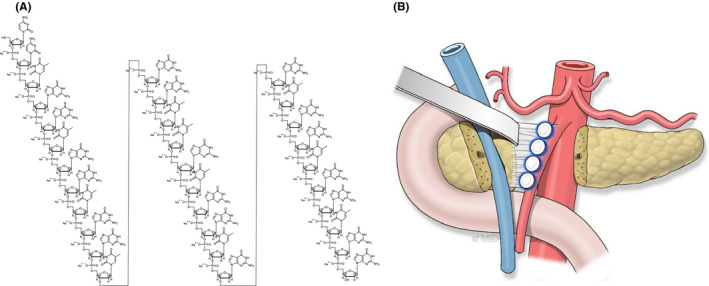
A, The molecular structure of the pancreatic cancer–specific aptamer–conjugated chemotherapeutic agent–loaded collagen patch, which is supposed to continuously deliver a localized and efficient dose of anticancer agent. B, A future model for the potential application of aptamer‐conjugated gemcitabine–loaded atelocollagen patch in clinical practice after pancreatic cancer surgery
In the present study, we investigated the anticancer effect of the aptamer‐conjugated gemcitabine‐loaded atelocollagen patch in a pancreatic cancer patient–derived xenograft (PDX) model to propose a future potential adjuvant surgical strategy during curative pancreatic resection for pancreatic cancer. (Figure 1B).
2. MATERIALS AND METHODS
2.1. Aptamer‐based targeting of pancreatic cancer
Aptamers are short, single‐stranded DNA or RNA molecules that can selectively bind to a specific target, including proteins, peptides, carbohydrates, small molecules, toxins, and even live cells. Nucleolin is a multifunctional protein distributed in the nucleolus, cytoplasm, and on the cell membrane. Some studies mentioned its role in cancer cells for carcinogenesis, proliferation, and metastasis and noticed that its inhibition can strongly impair the growth of primary tumors and liver metastasis in an orthotropic mouse model of pancreatic ductal adenocarcinoma. 1 , 2 We evaluated the degree of nucleolin protein expression in pancreatic cancer cell lines compared with normal tissues via Western blot and estimated its potential as a target of aptamer.
2.2. Preparation of the patch
The patch is an implant composed of type 1 atelocollagen containing a (2’‐deoxy‐2’,2’‐difluoro)C‐(2’‐deoxy‐2’,2’‐difluoro)C‐[TGG]m[TTG][TGG]n that is released throughout a period of months into a tumor. Type I atelocollagen was supplied in dried form by Interoligo. Type I atelocollagen was extracted from porcine skin using acetic acid in the presence of pepsin and then purified by dialysis against distilled water and lyophilized. (2’‐Deoxy‐2’,2’‐difluoro)C‐(2’‐Deoxy‐2’,2’‐difluoro)C‐[TGG]4[TTG][TGG]5 was synthesized by Interoligo Corp.
AS1411 is a quadruplex‐forming oligodeoxynucleotide that binds to nucleolin as an aptamer, but its mechanism of action is not completely understood. 3 However, since its first introduction, 4 various studies reported the anticancer effect of AS1411 in various types of cancers. 5 , 6 , 7 , 8 We used a modified type of this aptamer (AS1411) demonstrated by C’C’T‐AS1411 (IO101) and C’C’T‐AS1411‐TGG (IO101L) (see Figure 2). We evaluated the effect of stability and solubility in C’C’T‐AS1411‐TGG (IO101L) compared with C’C’T‐AS1411 (IO101). By adding C’C’T to the 5’ end and Guanine‐Guanine‐Thymine (GGT) to the 3’ end of AS1411, stability and solubility were improved compared with adding C’C’T only at the 5’ end of AS1411. The negatively charged aptamer causes complexation with the cationic atelocollagen. There is a difference in solubility between C’C’T‐AS1411 (IO101) and C’C’T‐AS1411‐TGG (IO101L) when mixed with atelocollagen. A formulation has been developed in which C’C’T‐AS1411‐TGG (IO101L) is better mixed with atelocollagen than C’C’T‐AS1411 (IO101), and the drug is evenly distributed.
FIGURE 2.
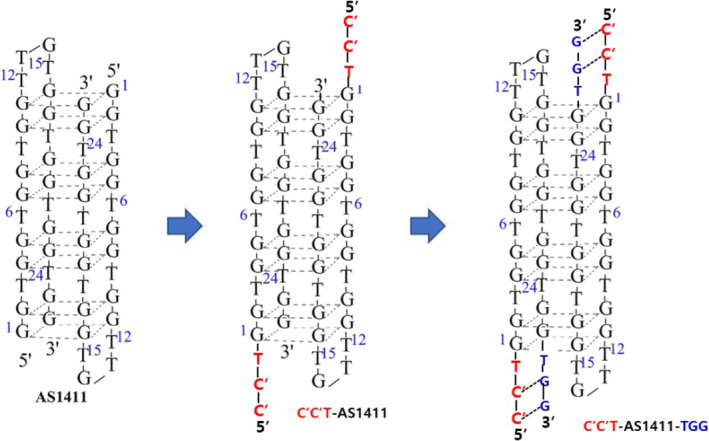
The molecular structures of a modified type of aptamer (AS1411) demonstrated by C’C’T‐AS1411 (IO101) and C’C’T‐AS1411‐TGG (IO101L)
Each atelocollagen‐modified aptamer formulation was prepared by gently mixing aqueous atelocollagen with a solution containing a defined concentration of modified aptamer. Atelocollagen and a solution containing a defined concentration of modified aptamer were well mixed, poured into an appropriate mold, and then freeze‐dried to prepare a patch having a desired shape and size. The modified aptamer in the patch was quantitatively analyzed by FPLC. The atelocollagen concentration of the patch was quantified using the SircolTM collagen assay kit.
2.3. Preclinical trial agents
The following agent, patch type gemcitabine targeted pancreatic tumor were evaluated at Interoligo Corporation & Korean Union pharmaceutical company for the in vivo antitumor efficacy as a pre‐clinical assay in established human pancreatic cancer PDXs mice.
2.4. Evaluation of the uptake of nucleolin‐aptamer–conjugated gemcitabine (Gem‐Aptamer) by pancreatic cancer cell lines (BXPC)
Based on the result of the nucleolin expression of the pancreatic cancer cell lines, we selected the BXPC pancreatic cancer cell line as potential target of aptamer. BXPC cells were cultured in a Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum and 1% penicillin‐streptomycin at 37°C in a 5% carbon dioxide environment. To confirm the intracellular uptake of Gem‐Aptamer, it was stained with Cy5 (excitation: 649 nm, emission: 670 nm). After treatment with 10 μM of Gem‐Aptamer, intracellular uptake was observed using a fluorescence microscope for each time period.
2.5. Evaluation of apoptosis efficacy of Gem‐Aptamer using Hoechst in the BXPC cell line
Cultured BXPC cells were dispensed into an eight‐well chamber with 1 × 104 cell/well and stabilized for 24 hours. Then, Gem‐Aptamer (1, 10 μM) was treated for 72 hours. After washing with phosphate‐buffered saline (PBS), cells were fixed with 4% formalin and treated with 0.1% triton X‐100. After that, blocking was performed with 5% bovine serum albumin (BSA), and anti‐Ki67 was incubated at 25°C for 1 hour, followed by the attachment of a specific secondary antibody. After washing with PBS and treated with Hoechst for 3 minutes, the reaction was observed using a florescence microscope.
2.6. Analysis of the physical characteristics of the patches and releasing profile in vitro
We analyzed the physical characteristics of the patches. Patches were coated with Pt (5 nm) by an ion coater (LEICA EM ACE600) so that disc‐shaped patches were examined and photographed with field emission scanning electron microscopy (FE‐SEM; Merlin, Carl ZEISS) at 2 kV. This experiment was done with the technical support of Avison Biomedical Research Center (ABMRC). The releasing concentration of aptamer‐conjugated gemcitabine from patches was measured indirectly by the concentration of aptamer. First, each patch was soaked in 1 ml PBS. Then, the tubes were incubated in a 37°C incubator until the end of the measurement. The solution was taken and loaded onto Nanodrop 2000 (ThermoFisher Scientific) because gemcitabine‐conjugated aptamer is a single‐stranded oligonucleotide. The cumulative release profile of aptamer‐conjugated gemcitabine from the patch was monitored up to 55 days.
2.7. Establishment of a subcutaneous pancreatic cancer PDX mouse model
After preparation of the patches, animal studies were carried out both in male nonobese diabetic/severe combined immunodeficient, NOD/Shi‐scid, IL‐2RγKO mice (NOG mice®) (Central Lab Animal Inc., Saeronbio Inc) and female nu/nu athymic mice (Orientbio), maintained under pathogen‐free conditions and a 12‐hour light/12‐hour dark cycle. Ten fresh pancreatic tumor specimens archived from originally resected patient tumor at the time of surgery, with informed written patient consent (Institutional Review Board No.4–2017–0594), were delivered within 1 hour on ice‐cold RPMI media tube to the Patient‐Derived Tumor Xenograft (PDTX) clean bench according to the PDX Standing operating procedure (SOP) of the Department of Laboratory Animal Resources (Yonsei Biomedical Research Institute). The specimens were washed with ice‐cold PBS, and the non‐necrotic fragments were cut into small pieces (2 × 2 × 2 mm), removing any blood coat or lipid part, and coated with Matrigel on petri dish. The single pieces were subcutaneously implanted into the right and left flank side of 6‐week‐old mice using Precision Trochar 10 gauge (MP182, Innovative Research of America) and sealed with 5–0 suture (VCP490G, ETHICON). When successfully taken up, the tumor size reached up to 1500 mm³ (donor mice, F1).
F1 tumors were subsequently transplanted to cohort mice (F2) in the same way, and the rest of the tumor was stored at freezing vial containing 5% dimethyl sulfide/95% fetal bovine serum in liquid N2 as a stock. To reach palpable tumors (approximately 150~200 mm³ of volume), it took approximately 50 days for each passage, and finally F2 mice were prepared, for drug efficacy test. Once the tumor reached a size of a palpable tumor mass (average size = 266.5 ± 58.0 mm3), mice (n = 8~13/group, n = >4/patient) were divided randomly into six groups: group 1, no‐treatment control; group 2, receiving gemcitabine (100 ug) suspended in PBS given intraperitoneally (IP) to mice one time for 4 weeks; group 3, receiving aptamer‐gemcitabine (100 ug) suspended in PBS given IP to mice one time for 4 weeks; groups 4, 5, and 6 implanted with three kinds of patches. The control mice that received no treatment were also included for comparison (Supplementary data 1).
2.8. Assessment of chemotherapeutic efficacy in a human pancreatic PDX mouse model
The effect of systematical administration of patch loaded drugs was checked as in the other group. F2 PDX mice were divided into two drug treatment groups (n = 8/group, n = 4/patient). A gemcitabine stock solution (472 ug/ml) and an aptamer‐gemcitabine stock solution (40 ug/100ul) were made and each solution was intraperitoneally (IP) injected into each mice group to a final concentration of 110 ug per mice. Tumor sizes and body weights were recorded two to three times per week for 1 month; the tumor dimensions were measured using a caliper (Mitutoyo). Tumor volumes were calculated using the following formula: tumor volume = (length × width2)/2.
2.9. Evaluation of antitumor efficacy of the patch in human pancreatic PDXs model
Animals were grouped randomly (four to nine mice per group, with two patient tumors) into no‐treatment control group; treatment group treated with IP gemcitabine injection (IP‐GEM) or aptamer‐conjugated gemcitabine (APT:GEM); or transplant with three kinds of patches: atelocollagen‐aptamer‐gemcitabine (patch I), atelocollagen‐inactive aptamer‐gemcitabine (patch II), and atelocollagen‐gemcitabine (patch III). Patch I consisted of atelocollagen‐active aptamer‐gemcitabine complexes, which we expect the maximal antitumor effect. In the control group, patch II consisted of atelocollagen‐inactive aptamer‐gemcitabine and inactive aptamer composed of C‐rich oligonucleotide (CRO), in which all “G”s were replaced with “C”s in the G‐rich oligonucleotide (GRO) of active aptamer. Patch III had only atelocollagen‐gemcitabine complexes without aptamer. After skin incision, subcutaneous blunt dissection between skin and tumor was performed to make some room for implanting local patches (Supplementary data 2).
Tumor size was measured three times per week by caliper measurements (Mitutoyo, Absolute AOS Digmatic), and volumes were calculated as above. Tumor growth in drug‐treated animals was compared with that in vehicle‐treated mice and represented as tumor growth rate (tumor volume at time/initial tumor volume). The statistical significance of the data was evaluated by IBM® SPSS® Statistics version 23. All results are presented as mean ± standard deviation. Mann‐Whitney’s U test was applied to compare the continuous variables according to different groups. P‐value less than 0.05 was considered significant.
Mice were sacrificed 1 month after attaching patches, and samples of tissues and whole blood were taken. Extracted tumor was weighed on a balance, and its photo was recorded in a Nikon digital camera. Fresh tumor tissue was placed for 24 hours in 10% neutral‐buffered formalin immediately after harvest and then embedded in paraffin. Thin sections (4 μm) were prepared, and hematoxylin and eosin (H&E) staining was performed by the technical support of ABMRC. For immunohistochemistry, the unstained tissue sections were incubated with anti‐nucleolin antibody at 1:200 diluted ratio (ab22758). After completing the incubation, the slides were observed under a phase‐contrast microscope.
2.10. TUNEL assay and histological analyses
In order to evaluate the anticancer effect of the patch, we performed a TUNEL assay. Paraffin was removed from the paraffin‐sectioned slides prior to the labeling reaction, and the slides were processed by in situ labeling procedure with TdT labeling (Catalog #4810‐30‐K, Trevigen)/biotinylated deoxyuridine (Roche Diagnostics). After reaction, the tissue sections were counterstained and prepared for microscopy viewing. Apoptotic cells will exhibit increased methyl green uptake containing fragmented nuclear chromatin characterized by dark brown nuclear staining under phase‐contrast microscope. Other organs (liver, lung, kidney, spleen) tissues were analyzed for toxicity histologically. All organs/tissues were fixed in 10% neutral‐buffered formalin for 24 hours in room temperature, embedded in paraffin, sectioned at a thickness of 4‐6 μm, and stained with H&E.
2.11. In vivo toxicity—hematology and histology
Blood samples were collected for hematologic determinations in BD Microtainer tubes with K2E (K²EDTA) as an anticoagulant and for toxicity test in serum‐collecting BD Microtainer chemistry tubes Serum‐Separate Tube (SST). Hematologic determinations included complete blood cell count (CBC)—white blood cells (WBCs), red blood cells (RBCs), hemoglobin concentration, hematocrit content, platelet count (PLT), differential leucocyte count (neutrophils, lymphocytes, monocytes), and reticulocyte count—using a HEMAVET 950FS analyzer (DREW Scientific). This clinical pathological assay was done with the technical support of the Department of Laboratory Animal Resources (Yonsei Biomedical Research Institute.
2.12. Institutional review board approval
All protocols and procedures including human subjects and vertebrate animals were approved by the Institutional Review Board of Severance Hospital (IRB No.4–2017–0594) and the Institutional Animal Care and Use Committee of Yonsei University College of Medicine (IACUC No. 2017–0276).
3. RESULTS
To evaluate nucleolin as a potential target in human pancreatic cancer, we analyzed nucleolin protein expression in multiple human pancreatic cancer cell lines by Western blot. All of the pancreatic cancer cell lines including HPaSC, ASPC1, Capan1, Miapaca, and Panc1 showed high nucleolin expressions, but the normal human pancreatic duct epithelial (HPDE) cell line and control group showed no expression (Figure 3).
FIGURE 3.
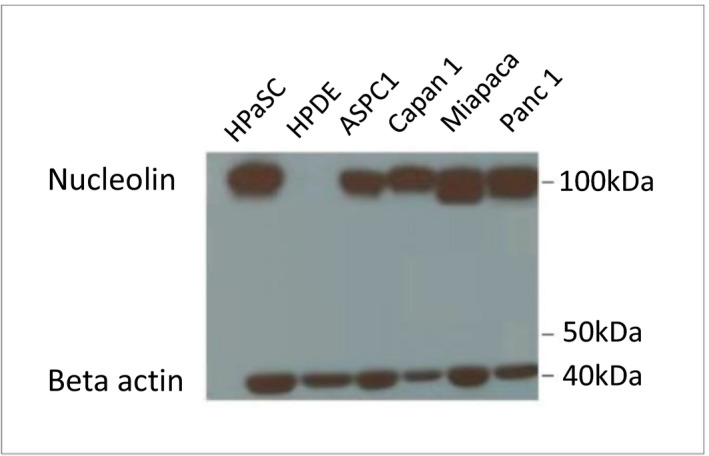
Analysis of nucleolin protein expressions in multiple human pancreatic cancer cell lines by Western blot. All of the pancreatic cancer cell lines including HPaSC, ASPC1, Capan1, Miapaca, and Panc1 showed high nucleolin expressions (100 kDa), but the normal pancreatic duct cell line (HPDE) and control group showed no expression (beta actin: 42 kDa)
Among the pancreatic cancer cell lines, we selected BXPC cell lines as potential target for Gem‐Aptamer. We evaluated the intracellular uptake of Gem‐Aptamer with Cy5 florescence and confirmed that Gem‐Aptamer enters the cytoplasm after about 1 hour of administration and is delivered into the nucleus within 3 hours. However, this does not mean that the efficacy of the drug appears after 3 hours (Supplementary data 3—video file).
Ki67 is a factor involved in cell proliferation and Hoechst stands for DNA. Gem‐Aptamer decreased the expression level of Ki67 in a concentration‐dependent manner in BXPC cells. The amount of DNA was also reduced. Assuming that the fluorescence expression level of Ki67 compared with Hoechst was 100% in the untreated group, the expression level was reduced by 43.32 ± 3.96% in 1 μM of Gem‐Aptamer and 27.06 ± 9.32% in 10 μM (Figure 4).
FIGURE 4.
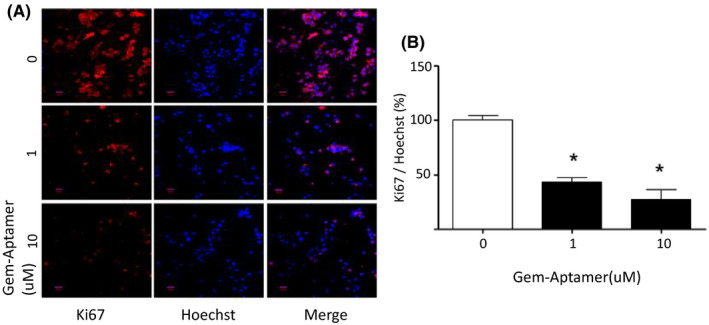
Ki67 and Hoechst suppression according to the concentration of nucleolin‐aptamer–conjugated gemcitabine (Gem‐Aptamer). A, Gem‐Aptamer decreased the expression level of Ki67 in a concentration‐dependent manner in BXPC cells. B, The amount of DNA was also reduced. Assuming that the fluorescence expression level of Ki67 compared with Hoechst was 100% in the untreated group, the expression level was reduced by 43.32 ± 3.96% in 1 μM of Gem‐Aptamer and 27.06 ± 9.32% in 10 μM
Based on the in vitro study, we developed a locally applicable aptamer‐conjugated gemcitabine‐loaded atelocollagen patch. The external morphology of this local drug delivery system has a white‐colored atelocollagen‐based disc‐like structure. The size of an individual atelocollagen disc is 1 cm in diameter and 1 mm in depth (Figure 5A). The microstructure was determined by SEM. It harbors variable‐sized microporous structures, which might be appropriate for embedding Gem‐Aptamer inside the disc (Figure 5B,C). In the analysis of the releasing profile of the embedded chemotherapeutic agent, the 265‐nm UV absorbance of serially diluted gemcitabine showed a linear correlation with statistical significance (R 2 = 0.931, p < 0.001) (Figure 5D). The correlation between UV absorbance and released gemcitabine and nucleolin‐aptamer concentration was assessed by collecting the media supernatants of the patch at different time points. Most of the Gem‐Aptamer was released within 1 day, and continuous drug release was observed for up to 28 days (Figure 5E,F).
FIGURE 5.
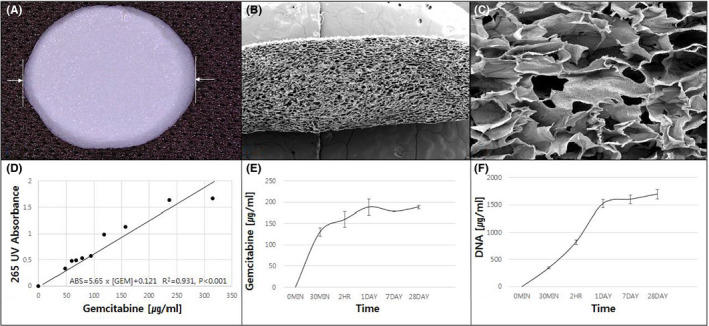
‐ Physical characteristics of the atelocollagen patch and embedded drug–releasing profile in vitro. A, The external morphology of local drug delivery system has white‐colored atelocollagen‐based disc‐like structure. The size of an individual atelocollagen disc is 1 cm in diameter and 1 mm in depth (B, C). The microstructure of the disc by the scanning electron microscopy (SEM). It has variable‐sized microporous structures, which might be appropriate for embedding nucleolin‐aptamer–conjugated gemcitabine (D). The 265‐nm UV absorbance and serially diluted gemcitabine showed a linear correlation with statistical significance (E, F). Most of the nucleolin‐aptamer–conjugated gemcitabine was released within 1 day, and continuous drug release was observed for up to 28 days
A PDX model was successfully established from the resected pancreatic head cancer of a 65‐year old female patient who underwent laparoscopic pancreaticoduodenectomy. Pathologic examination revealed that it was a 3.2‐cm, poorly differentiated pancreatic ductal adenocarcinoma, which was associated with perineural and frequent lymphovascular invasion. Among seven retrieved lymph nodes, one metastatic lymph node was found (AJCC 8th T2N1M0, Stage IIB). When comparing the histological features of the PDX and its original primary tumor by H&E and nucleolin immunostaining, gross histological similarities were found between primary tumors and PDX tumors (Supplementary data 4). The sizes of the implanted pancreatic cancer were found to be increasing with different tumor growth rates according to individual groups. Among them, the patch I group (Gem‐Aptamer–loaded atelocollagen patch) showed the most significant reduction of tumor growth rate, compared with the no‐treatment group. It was found that there were statistically significant differences in tumor growth rate between the no‐treatment group and the patch I group on the 7th day (1.89 ± 0.29 vs 0.92 ± 0.29, p = 0.034), on the 10th day (2.79 ± 1.09 vs 1.04 ± 0.36, p = 0.034), and on the 14th day (4.11 ± 2.21 vs 1.09 ± 0.68, p = 0.034). These statistical differences became attenuated to marginal significance on the 21th day (5.60 ± 3.88 vs 2.82 ± 1.23, p = 0.059) and on the 28th day (6.38 ± 3.09 vs 2.14 ± 1.48, p = 0.059). However, other treatment groups were not found to show significant reduction in tumor growth rate (0.05 < p < 0.1, Figure 6). No tumor necrosis or apoptotic process was found in the no‐treatment group (Figure 7A,B). However, apoptotic process was identified in the IP‐GEM group, showing that intratumoral TUNEL‐positive cancer cells were found in the implanted cancer tissue (Figure 7C,D). To the contrary, the patch I group also showed significant apoptotic process, but TUNEL‐positive cancer cells were found along the superficial layer of the tumor surface, where the Gem‐Aptamer–loaded atelocollagen patch had been applied (Figure 7E,F). Under microscopic examination, the patch I group showed no microscopic evidence suggesting potential toxicity, such as inflammation or necrotic changes, in liver, lung, kidney, and spleen tissue (Figure 8). In blood laboratory examination, no leukopenia, no anemia, and no neutropenia were observed in the patch I group, which was in contrast to the IP‐GEM group associated with the systemic effect of the chemotherapeutic agent, gemcitabine, such as leukopenia (3.2 ± 2.9 vs 5.4 ± 2.9, p = 0.028), lower hemoglobin level (10.3 ± 4.6 vs 18.5 ± 11.9, p = 0.010), and neutropenia (0.76 ± 0.71 v. 2.69 ± 2.66, p = 0.010; Table 1).
FIGURE 6.
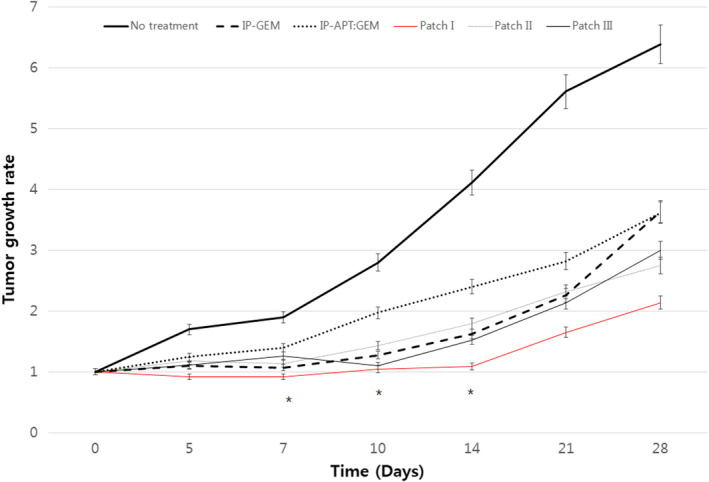
Therapeutic effect of the nucleolin‐aptamer–conjugated gemcitabine–loaded atelocollagen patch in pancreatic cancer patient–derived xenograft model. The size of the implanted pancreatic cancer was found to be increasing with different tumor growth rates according to individual groups. Among them, the patch I group (necleolin‐aptamer–conjugated gemcitabine–loaded atelocollagen patch) showed the most significant reduction of tumor growth rate compared with the no‐treatment group. *Mann‐Whitney U, p < 0.05
FIGURE 7.
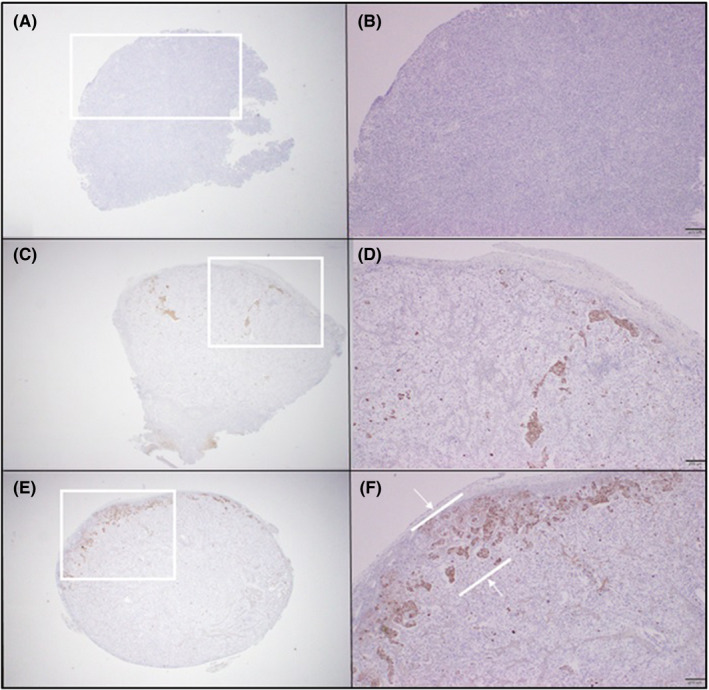
Anticancer effect of the patch in TUNEL assay. No tumor necrosis or apoptotic process was found in no treatment group (A, B). However, apoptotic process was identified in the intraperitoneal gemcitabine injection (IP‐GEM) group, showing that intratumoral TUNEL‐positive cancer cells were found in the implanted cancer tissue (C, D). Patch I group also showed significant apoptotic process, but TUNNEL positive cancer cells were found along the superficial layer of the tumor surface, where the patch was applied (E, F)
FIGURE 8.
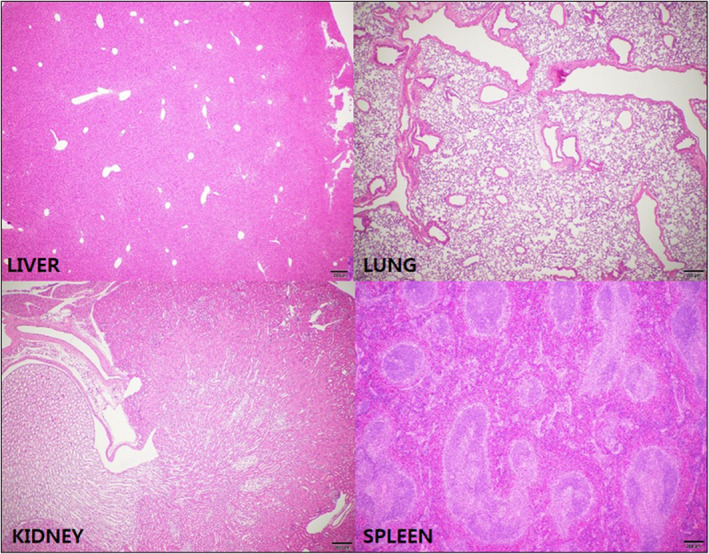
Microscopic examination of potential toxicity in liver, lung, kidney, and spleen tissue. The patch I group showed no microscopic evidence suggesting potential toxicity, such as inflammation or necrotic changes in liver, lung, kidney, and spleen tissue
TABLE 1.
Hematologic change between IP‐GEM and patch I
| Parameter (units) | Control | IP‐GEM | Patch I | p‐value 1 | p‐value 2 |
|---|---|---|---|---|---|
| WBC (X1000/㎕) | 5.8 ± 2.5 | 3.2 ± 2.9 | 5.4 ± 2.9 | 0.679 | 0.028 |
| HB (g/dL) | 16.5 ± 1.3 | 10.3 ± 4.6 | 18.5 ± 11.9 | 0.768 | 0.010 |
| PLT (X1000/㎕) | 1265.8 ± 477.5 | 684.1 ± 246.3 | 770.4 ± 284.1 | 0.040 | 0.447 |
| Neutrophil (X1000/㎕) | 1.6 ± 0.9 | 0.76 ± 0.71 | 2.69 ± 2.66 | 0.594 | 0.010 |
| Lymphocyte (X1000/㎕) | 3.6 ± 1.6 | 1.87 ± 1.74 | 7.97 ± 18.23 | 0.310 | 0.113 |
Abbreviations: IP‐GEM, intraperitoneal gemcitabine injection; PLT, platelet count; WBC, white blood cell; HB, hemoglobin.
Mann‐Whitney U between control and patch I.
Mann‐Whitney U between IP‐GEM and patch I.
4. DISCUSSION
Recently, effective chemotherapeutic agents, such as FOLFIRINOX 9 and nanoalbumin‐bound paclitaxel (Nab‐Paclitaxel), 10 for pancreatic cancer have been introduced to show improved survival outcomes. Therefore, the development of a new treatment concept to potentiate the curative effect of margin‐negative resection without impairing the patient's safety is mandatory.
As an effort to enhance the therapeutic effect of margin‐negative pancreatectomy, we developed locally applicable a Gem‐Aptamer–loaded atelocollagen patch to continuously release an efficient dose of gemcitabine specifically against potential microscopic residual cancer cells left around surgical fields. Collagen is widely used for regenerative therapy and pharmaceutical applications. The safety of collagen has been demonstrated, and it is used in many clinical practices of general surgery for resorbable surgical suture, hemostatic agents, and wound healing. 11 , 12 , 13 , 14 Especially, atelocollagen administered into the human body is not dissolved immediately, but can exist for a long time, which is advantageous to a sustained release of drug. 15 In addition, Hirasawa, et al. 16 confirmed atelocollagen is eliminated by a natural process of degradation and absorption, similar to endogenous collagen. These results suggest atelocollagen is safe and can be appropriate as new drug delivery system to enhance the clinical potency of the target drug.
The nucleolin is known to be highly expressed in pancreatic cancer 17 and can be a novel target for anticancer therapy as demonstrated by the effects of several nucleolin‐targeting molecules. 18 The present study also confirmed that a high level of nucleolin protein is expressed in pancreatic cancer, and survival curves differed according to its expression, which is thought to be one of the effective and specific guidance to pancreatic cancer cell for enhancing the therapeutic efficacy of chemotherapeutic agents.
Gemcitabine has been recognized by many oncologists as the first‐line drug to treat pancreatic cancer. 19 , 20 , 21 Therefore, the Gem‐Aptamer–loaded atelocollagen patch is supposed to continuously release an efficient dose of gemcitabine, providing potential anticancer effect for microscopic residual cancer with minimizing the systemic adverse effect of chemotherapeutic agents. Our present results support these hypotheses. It was observed that only the patch I group (Gem‐Aptamer–loaded atelocollagen patch) showed statistically significant tumor growth–inhibiting effect when compared with the control group (Mann‐Whitney U, p < 0.05). In addition, apoptotic process was clearly noted along the surface of tumor where patch I was placed over, showing effective drug delivery and anticancer effect against pancreatic cancer cells near the patch. This finding strongly suggests the Gem‐Aptamer–loaded atelocollagen patch can play an important adjuvant role to enhance the oncologic significance of potentially margin‐negative resection in treating pancreatic cancer. In addition, compared with the IP‐GEM group, systemic adverse effects, such as leukopenia, neutropenia, and anemia were not associated with the patch I group, suggesting potential safety and clinical feasibility of intraoperative local application in the case of curative pancreatectomy for pancreatic cancer.
Recently, there has been important progress in developing an implantable chemotherapy system in treating pancreatic cancer. Byrne et al. 22 , 23 fabricated implantable iontophoretic devices and tested the local iontophoretic delivery of FOLFIRINOX in an orthotropic pancreatic cancer PDX model, showing treatment with device FOLFIRINOX was associated with significant inhibitory effect in tumor growth compared with i.v. FOLFIRINOX. Logan et al. 24 also developed implantable double‐layered poly (d,l‐lactic‐co‐glycolic acid) (PLGA) cylinders for the sequential release of gemcitabine in combination with oseltamivir phosphate (OP) over 30 days, demonstrating effective reduction in cell viability of pancreatic cancer cell line PANC1 and its gemcitabine‐resistant variant for up to 15 days. Wade et al. 25 evaluated gemcitabine‐eluting wet‐spun polymeric fibers for localized drug delivery applications. They found that loaded gemcitabine was rapidly released within the first 10 hours followed by a sustained release over the next 134 hours, and time‐dependent inhibition of tumor spheroid growth and cell viability. Jun et al. 26 , 27 fabricated a poly‐L‐lactic acid–based 5‐FU–releasing patch by electrospinning and tested the oncologic effect of the combination of a local drug patch and systemic chemotherapy. This combination treatment resulted in increased tumor suppression effects that lasted longer, as well as increased survival rate, showing potential benchmarking for future clinical practice. Ramot et al. 28 introduced a biodegradable polymeric matrix local drug eluter (LODERTM), which can be a novel way to protect drugs against enzymatic degradation and to release small interfering RNA (siRNA) against G12D‐mutated KRAS (siG12D). In a preclinical study, they confirmed that there were no adverse reactions or abnormal laboratory tests, showing the safety of the delivered drug, waiting for future clinical application. Of note, Golan et al. 29 performed a 1/2a study in locally advanced pancreatic cancer (LAPC). In this study, patients were assigned to receive a single dose of siG12D‐LODERTM, and gemcitabine was given on a weekly basis following the siG12D‐LODERTM insertion until disease progression. They found frequent adverse events were of low grade in severity, and majority of the patients were in stable disease with decreasing CA 19–9. Median overall survival was 15.12 months, and the 18‐month survival was 38.5%. They concluded a combination of siG12D‐LODER™ and chemotherapy is well tolerated and safe and demonstrated a potential efficacy in patients with LAPC. Like these cases, variable local drug delivery systems combined with new potent chemotherapeutic agents are expected to emerge and be tested for future clinical application in treating pancreatic cancer.
Recently, Park et al. 5 reported a nucleolin‐targeted aptamer (APTA‐12) as pancreatic cancer–targeted anticancer agent and proved its efficacy in vitro and in vivo, by injection into the pancreatic cancer cell line in xenograft models. This study assumes and evaluates the effect of systemic administration of targeted anticancer agents. On the contrary, the current drug delivery system that we used (Gem‐Aptamer–loaded atelocollagen patch) was designed for localized administration of the target agent as adjuvant to curative pancreatectomy. In an analysis of the releasing profile of this patch, the plateau concentration of gemcitabine was around 150 ug/ml, whereas the mean blood concentration of systemic gemcitabine chemotherapy is reported to be about 15 ug/ml, which means more efficient drug dose was delivered to the target without systemic toxicity. To the best of our knowledge, this is the first study combining a nucleolin‐targeted anticancer agent and localized drug‐eluting patches.
This is a pilot study testing the potential anticancer effect of a Gem‐Aptamer–loaded atelocollagen patch just based on a small number of pancreatic cancer subcutaneous PDX models. In order to simulate clinical application in resectable pancreatic cancer, the anticancer effect of the Gem‐Aptamer–loaded atelocollagen patch should be tested in a partial tumor resection–orthotropic PDX model in near future. Recently, Groot et al. 30 demonstrated the oncologic impact of patterns and the timing of recurrence following pancreatectomy for pancreatic cancer, suggesting unique biological characteristics of pancreatic cancers leading to distinct patterns of recurrence. If we are able to predict the potential local recurrence site based on primary tumor location, 31 , 32 this local anticancer drug delivery system will be very useful in clinical practice of radical pancreatectomy. Considering approximately the half of the patients are found in locally advanced stage of cancer, development of a “sol‐gel” type of atelocollagen with Gem‐Aptamer will expand cases for potential application by endoscopic ultrasound‐guided approach in near future. 29 , 33 In addition, nucleolin was also found to be highly expressed in other malignancies such as lung cancer or brain tumor, suggesting the possibility of therapeutic application to these lethal diseases in near future.
In summary, we demonstrated the potential anticancer effect of a Gem‐Aptamer–loaded atelocollagen patch in a pancreatic cancer subcutaneous PDX model. It showed significant tumor growth inhibitory effect and no adverse systemic side effects. This implantable ApDC system is thought to be a new surgical strategy to augment the oncologic significance of margin‐negative resection in treating pancreatic cancer in near future. Further study is mandatory.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Industrial Strategic Technology Development Program/Bio‐Industry Core Technology Development Business (Promotion of Promising Bio IP Business) New Project (10078393) (Development of a drug‐delivery system that can sustain the release of drugs for more than one month and direct drug delivery therapeutic efficacy evaluation in pancreatic cancer by using the atelocollagen‐mediated aptamer‐drug conjugate stabilization source technology) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Hong SS, Lee S, Lee SH, et al. Anticancer effect of locally applicable aptamer‐conjugated gemcitabine‐loaded atelocollagen patch in pancreatic cancer patient–derived xenograft models. Cancer Sci. 2022;113:1752–1762. doi: 10.1111/cas.15318
Jung Hwan Lee and Chang Moo Kang equally contributed as corresponding authors.
Seung Soo Hong and Sena Lee equally contributed as co–first authors.
Contributor Information
Jung Hwan Lee, Email: jhlee@interoligo.com.
Chang Moo Kang, Email: cmkang@yuhs.ac.
REFERENCES
- 1. Chen Z, Xu X. Roles of nucleolin. focus on cancer and anti‐cancer therapy. Saudi Med J. 2016;37:1312‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilles ME, Maione F, Cossutta M, et al. Nucleolin targeting impairs the progression of pancreatic cancer and promotes the normalization of tumor vasculature. Cancer Res. 2016;76:7181‐7193. [DOI] [PubMed] [Google Scholar]
- 3. Reyes‐Reyes EM, Teng Y, Bates PJ. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin‐dependent mechanism. Cancer Res. 2010;70:8617‐8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957‐2962. [DOI] [PubMed] [Google Scholar]
- 5. Park JY, Cho YL, Chae JR, et al. Gemcitabine‐incorporated G‐quadruplex aptamer for targeted drug delivery into pancreas cancer. Mol Ther Nucleic Acids. 2018;12:543‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvalho J, Paiva A, Cabral Campello MP, et al. Aptamer‐based targeted delivery of a G‐quadruplex ligand in cervical cancer cells. Sci Rep. 2019;9:7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehrnia SS, Hashemi B, Mowla SJ, Nikkhah M, Arbabi A. Radiosensitization of breast cancer cells using AS1411 aptamer‐conjugated gold nanoparticles. Radiat Oncol. 2021;16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghahremani F, Shahbazi‐Gahrouei D, Kefayat A, Motaghi H, Mehrgardi MA, Javanmard SH. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Advances. 2018;8:4249‐4258. [Google Scholar]
- 9. Lambert A, Gavoille C, Conroy T. Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Therap Adv Gastroenterol. 2017;10:631‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giordano G, Pancione M, Olivieri N, et al. Nano albumin bound‐paclitaxel in pancreatic cancer: current evidences and future directions. World J Gastroenterol. 2017;23:5875‐5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chvapil M, Kronenthal L, Van Winkle W, Jr . Medical and surgical applications of collagen. Int Rev Connect Tissue Res. 1973;6:1‐61. [DOI] [PubMed] [Google Scholar]
- 12. Baik SH, Kim JH, Cho HH, Park SN, Kim YS, Suh H. Development and analysis of a collagen‐based hemostatic adhesive. J Surg Res. 2010;164:e221‐e228. [DOI] [PubMed] [Google Scholar]
- 13. Suh DS, Lee JK, Yoo JC, et al. Atelocollagen enhances the healing of rotator cuff tendon in rabbit model. Am J Sports Med. 2017;45:2019‐2027. [DOI] [PubMed] [Google Scholar]
- 14. Udhayakumar S, Shankar KG, Sowndarya S, Rose C. Novel fibrous collagen‐based cream accelerates fibroblast growth for wound healing applications: in vitro and in vivo evaluation. Biomater Sci. 2017;5:1868‐1883. [DOI] [PubMed] [Google Scholar]
- 15. Sano A, Maeda M, Nagahara S, et al. Atelocollagen for protein and gene delivery. Adv Drug Deliv Rev. 2003;55:1651‐1677. [DOI] [PubMed] [Google Scholar]
- 16. Hirasawa T, Sano A, Fujioka K, et al. Biodegradability of ‘minipellet’, a new drug formulation using atelocollagen as a drug carrier material, in the rhesus monkey. Biomed Res. 1997;18:149‐159. [Google Scholar]
- 17. Peng L, Liang J, Wang H, et al. high levels of nucleolar expression of nucleolin are associated with better prognosis in patients with stage ii pancreatic ductal adenocarcinoma. Clin Cancer Res. 2010;16:3734‐3742. [DOI] [PubMed] [Google Scholar]
- 18. Gilles M‐E, Maione F, Cossutta M, et al. Nucleolin targeting impairs the progression of pancreatic cancer and promotes the normalization of tumor vasculature. Can Res. 2016;76:7181‐7193. [DOI] [PubMed] [Google Scholar]
- 19. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative‐intent resection of pancreatic cancera randomized controlled trial. JAMA. 2007;297:267‐277. [DOI] [PubMed] [Google Scholar]
- 20. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil‐based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019‐1026. [DOI] [PubMed] [Google Scholar]
- 21. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073‐1081. [DOI] [PubMed] [Google Scholar]
- 22. Byrne JD, Jajja MR, Schorzman AN, et al. Iontophoretic device delivery for the localized treatment of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2016;113:2200‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Byrne JD, Jajja MRN, O'Neill AT, et al. Impact of formulation on the iontophoretic delivery of the FOLFIRINOX regimen for the treatment of pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:991‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allison Logan S, Brissenden AJ, Szewczuk MR, Neufeld RJ. Combinatorial and sequential delivery of gemcitabine and oseltamivir phosphate from implantable poly(d, l‐lactic‐co‐glycolic acid) cylinders disables human pancreatic cancer cell survival. Drug Des Devel Ther. 2017;11:2239‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wade SJ, Zuzic A, Foroughi J, et al. Preparation and in vitro assessment of wet‐spun gemcitabine‐loaded polymeric fibers: towards localized drug delivery for the treatment of pancreatic cancer. Pancreatology. 2017;17:795‐804. [DOI] [PubMed] [Google Scholar]
- 26. Jun E, Kim SC, Lee CM, Oh J, Lee S, Shim IK. Synergistic effect of a drug loaded electrospun patch and systemic chemotherapy in pancreatic cancer xenograft. Sci Rep. 2017;7:12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shim IK, Yi HJ, Yi HG, et al. Locally‐applied 5‐fluorouracil‐loaded slow‐release patch prevents pancreatic cancer growth in an orthotopic mouse model. Oncotarget. 2017;8:40140‐40151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramot Y, Rotkopf S, Gabai RM, et al. Preclinical safety evaluation in rats of a polymeric matrix containing an siRNA drug used as a local and prolonged delivery system for pancreatic cancer therapy. Toxicol Pathol. 2016;44:856‐865. [DOI] [PubMed] [Google Scholar]
- 29. Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560‐24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267:936‐945. [DOI] [PubMed] [Google Scholar]
- 31. Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys. 2013;87:1007‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi SH, Kim HY, Hwang HK, Kang CM, Lee WJ. oncologic impact of local recurrence in resected pancreatic cancer and topographic preference in local recurrence patterns according to tumor location. Pancreas. 2020;49:1290‐1296. [DOI] [PubMed] [Google Scholar]
- 33. Levy MJ, Alberts SR, Bamlet WR, et al. EUS‐guided fine‐needle injection of gemcitabine for locally advanced and metastatic pancreatic cancer. Gastrointest Endosc. 2017;86:161‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


