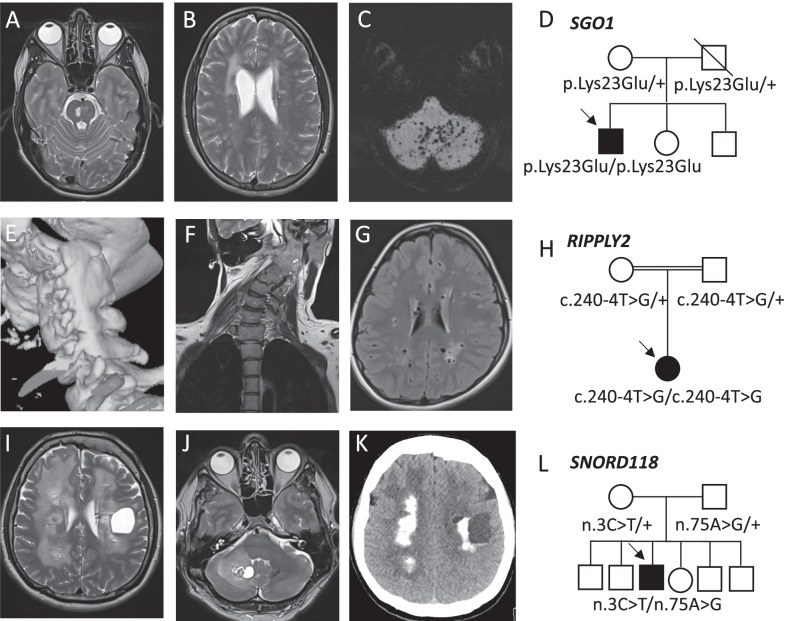
Fig. 1.
(Neuro)imaging of patients diagnosed with a rare disease which led to expansion of the phenotypic spectrum or which was associated with therapeutic changes. A, B T2- weighted MRI images of a patient diagnosed with CAID showing pontine and periventricular hyperintensities. C MRI image with maximum intensity projection (MIP) showing diffuse cerebellar microbleeds. D Pedigree of the CAID patient. He is the first child of non-consanguineous parents with a homozygous pathogenic SGO1 variant (p.Lys23Glu). Both parents are heterozygotes. E 3D-reconstruction of CT-scan imaging of the cervical spine revealing a unilateral fusion of the cervical vertebrae C1-C6 in a patient diagnosed with spondylocostal dysostosis 6. F Coronal MRI image showing torticollis. G Axial MRI Flair image showing enlarged perivascular spaces and white matter lesions. H Pedigree of the patient with consanguineous parents and a homozygous RIPPLY2 variant (c.240-4T>G). I, J T2-weighted MRI images of patient 4 revealing the presence of a large cyst in the left cerebral and the right cerebellar hemisphere and diffuse periventricular white matter hyperintensities. K CT-scan axial section showing diffuse cerebral calcifications. L Pedigree of the LCC patient. He is the only affected child of non-consanguineous parents and is compound heterozygous for two variants in SNORD118 (n.3C > T; n.75A > G). Both parents were heterozygous for one pathogenic variant