Figure 1.
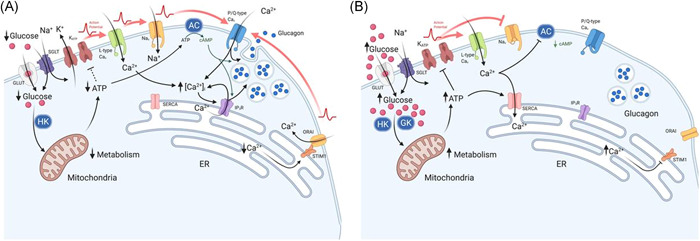
Effect of glucose on α‐cell Ca2+ and cAMP at (A) low glucose and (B) high glucose concentrations, which result in changes to glucagon secretion. (A) At low glucose, there is a low rate of glucose transport across the membrane via GLUT and SGLT proteins. Glucose is then converted to glucose‐6‐phosphate, by hexokinase 1 (HK), to generate ATP via glycolysis. A modest increase in the ATP:ADP ratio, leads to partial closure of KATP‐channels, maintaining the membrane potential at the point of allowing action potential (AP) generation: this enables the activation of L‐type Cav and Nav channels. The resulting high‐amplitude action potentials activate P/Q‐type channels, triggering exocytosis and glucagon release. In addition, adenylyl cyclase (AC) production of cAMP primes vesicles for exocytosis and potentiates Ca2+‐induced Ca2+ release from the endoplasmic reticulum (ER). Consequently, exocytosis of glucagon‐containing vesicles is high. The firing of action potentials can also be attributable to the activation of store‐operated Ca2+ entry (SOCE) via ORAI and STIM1, which is triggered by ER Ca2+ depletion due to low SERCA activity. (B) At high glucose concentrations, there is a high rate of glucose transport into the cytosol, leading to membrane depolarisation. This occurs as a result of increased ATP production via glycolysis, due to substrate abundance: high Km glucokinase (GCK) phosphorylates glucose to produce glucose‐6‐phosphate. High intracellular ATP results in complete closure of KATP‐channels and, consequently, membrane depolarisation. Membrane depolarisation can also be induced by the process of glucose transport through sodium/glucose co‐transporters (SGLTs): Na+ is co‐transported with glucose and thus provides a depolarising membrane conductance. Nav channels, at this membrane potential, become inactivated, whereas L‐type Cav channels remain activatable and contribute to action potential generation. However, action potential amplitude is reduced at high glucose concentrations (despite firing at a higher frequency) and are no longer able to activate P/Q‐type Cav channels, the exocytosis‐related Cav channel in α‐cells. The effect of membrane depolarisation and higher action potential firing frequency may also lead to increased cytosolic Ca2+ within domains adjacent to Ca2+‐sensitive adenylyl cyclase, inhibiting cAMP production and vesicle priming. However, higher ATP may also result in α‐cell membrane repolarisation. High ATP‐activated SERCA uptake of Ca2+ replenish ER Ca2+, deactivating ORAI‐mediated Ca2+ entry, resulting in suppression of α‐cell excitability and glucagon secretion. Red arrows indicate action potential propagation. Figure created using BioRender.com
