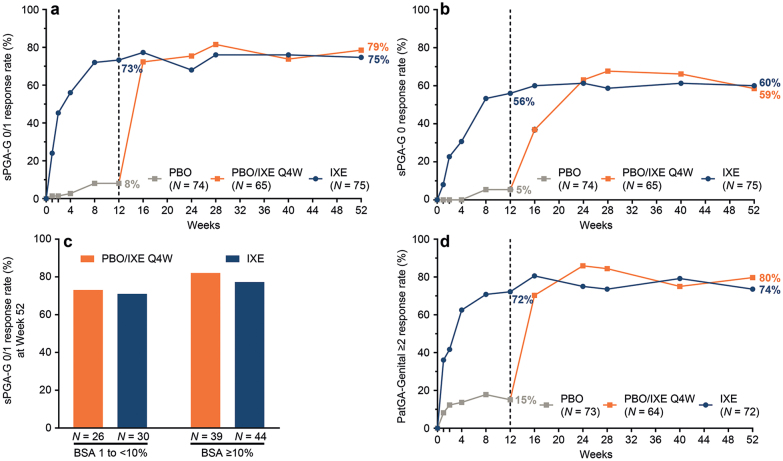
Fig. 1.
Genital psoriasis severity during 52 weeks (NRI) of treatment in IXORA-Q. (a) Proportion of patients achieving a static Physician’s Global Assessment of Genitalia (sPGA-G) score of 0 or 1 at each post-baseline visit. (b) Proportion of patients achieving complete resolution of genital psoriasis (sPGA-G 0) at each post-baseline visit. (c) Proportion of patients achieving a sPGA-G 0/1 at Week 52 among patients with lower (1 to <10%) and higher (≥10%) body surface area involvement at baseline. (d) Proportion of patients achieving at least a 2-point improvement in the Patient’s Global Assessment of Genital Psoriasis among patients with a baseline score of ≥2. (a-d) Results are summarized for the placebo (PBO) population (all patients randomized to placebo at Week 0) through Week 12, the PBO/IXE Q4W population (all patients randomized to placebo at Week 0 who entered the open-label treatment period and received at least 1 dose of ixekizumab) from Weeks 12 to 52, and the IXE population (all patients randomized to ixekizumab at Week 0) through Week 52.