Abstract
Microdialysis is a well-established technique for sampling of small molecules from the human skin, but larger molecules are more difficult to recover. Consequently, sampling feasibility must be evaluated before microdialysis is used in vivo. This report presents a tool for estimating the recovery of large biomarkers from human skin by microdialysis, using previously frozen human skin specimens as reservoirs for biomarker reference solutions. Recovery of the following 17 biomarkers was assessed: CCL27/CTACK, CXCL1/GROα, CXCL7/NAP-2, CXCL10/IP-10, EGF, GM-CSF, IFN-γ, IL-1α, IL-6, IL-8, IL-17, IL-22, IL-23, MIF, TNF-α, TSLP and VEGF. The relative skin recoveries of 13/17 biomarkers were successfully determined in the range 4.0–18.4%. Sampling in the skin reservoir model was not associated with probe leakage, as fluid recovery was stable, at between 80% and 110%. Furthermore, the skin reservoir model enabled studies and optimization of different parameters known to affect biomarker recovery, including flow rate and perfusate composition.
Key words: microdialysis, biomarker, cytokine, skin
Microdialysis is currently one of the only techniques available for sampling of exogenous and endogenous molecules from the interstitial fluid (1). The technique can be applied in studies of various tissues, including human skin, in vivo and ex vivo (2, 3). Traditionally, skin microdialysis has been used to recover small molecules, such as histamine (2, 4, 5), but the development of high molecular weight cut-off (MWCO) probes has enabled sampling of larger biomarkers, such as cytokines (6, 7). Compared with sampling of small molecules, larger biomarkers are more difficult to recover, due to their different physicochemical characteristics (6, 8–10). It is therefore critical to assess whether a given microdialysis setup enables recovery of the molecule(s) of interest, before the technique is used in studies of cutaneous events in vivo or ex vivo (1, 6, 11, 12).
Microdialysis sampling feasibility is usually investigated by measuring the relative recovery (also known as “extraction efficiency” (11)) of each target molecule. The relative recovery is defined as the analyte concentration in the dialysate divided by the concentration in the periprobe fluid, thus being the fraction of analyte crossing the membrane (2, 11). Many factors are known to affect the relative recovery, including the physicochemical properties of the analyte, membrane material, composition of the perfusate, membrane surface area and flow rate (1, 6). Consequently, the relative recovery of an analyte may vary greatly between different microdialysis setups.
SIGNIFICANCE
Microdialysis is a minimally-invasive technique to study skin reactions by sampling of molecules from the tissue; however, not all molecules are easily recovered. Therefore, we propose a new model to investigate the sampling efficiency of larger biomarkers: the skin reservoir model. This model simulates the sampling situation in living human beings by using previously frozen excised human skin as a reservoir for biomarker solutions. Thus, it serves as a tool to carefully validate and optimize sampling of the target molecules before the use of microdialysis; for example, in clinical studies.
So far, different non-standardized in vitro systems have been used to study the relative recovery of a broad range of biomarkers (for examples, see (11, 12)). Most setups estimate the relative recovery based on sampling while submerging the microdialysis probes in reference solutions with a known concentration of analyte (10–13). However, this approach is problematic when using high MWCO probes, as the increased pore-size of the membrane leads to issues with ultrafiltration. Many groups seeking to estimate the relative recovery of large biomarkers in vitro report an efflux of fluid from high MWCO probes, reducing or completely impeding fluid recovery (8, 10, 11, 14, 15), which is in line with our experience using similar in vitro setups.
We therefore present a new approach to evaluate the feasibility of recovering large biomarkers from human skin by microdialysis. Our new model, called “the skin reservoir model”, utilizes previously frozen human ex vivo skin as an alternative container for reference solutions, which are injected into the thawed skin specimens (Fig. 1). The ex vivo skin has undergone a freeze-thaw cycle to render it inactive, since no functional response is sought from the skin itself. The thawed skin serves only as a complex reference solution-reservoir, bearing closer resemblance to the in vivo cutaneous environment compared with sampling through reference solutions contained in a test tube or similar. When sampling has been carried out at the reference solution injection sites, the relative skin recovery of the analyte of interest can be calculated based on the dialysate concentration and the concentration of the reference solution injected.
Fig. 1.
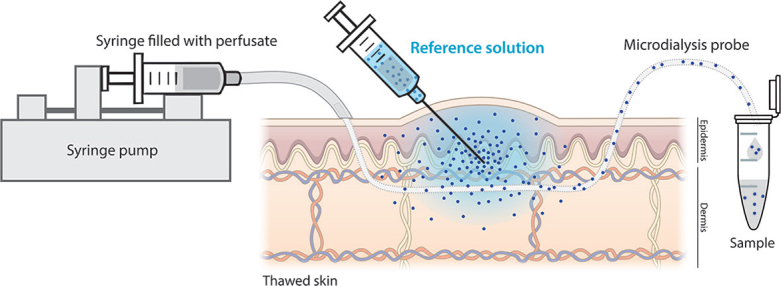
The skin reservoir model. Biomarker recovery can be studied in the skin reservoir model by injecting reference solutions into thawed skin specimens followed by microdialysis sampling through the injection sites. The relative skin recovery of the biomarker is estimated by measuring the fraction of the biomarker recovered into the dialysate.
This paper uses the skin reservoir model to study recovery of large biomarkers from human skin using microdialysis probes with a 3,000 kilodalton (kDa) MWCO. The study was carried out in 2 steps: first, 4 cytokines (interleukin (IL)-1α, IL-6, IL-17 and tumour necrosis factor (TNF)-α were selected as representative model analytes to characterize the skin reservoir model and to ensure that it fulfilled the theoretical principles of microdialysis sampling. Secondly, the panel was extended with 13 additional analytes: chemokine (C-C motif) ligand 27 (CCL27)/cutaneous T-cell-attracting cytokine (CTACK), chemokine (C-X-C motif) ligand (CXCL)1/growth-regulated oncogene α (GROα), CXCL7/neutrophil activating peptide-2 (NAP-2), CXCL10/interferon-γ-induced protein 10 (IP-10), epidermal growth factor (EGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ. IL-22, IL-23, macrophage migration inhibitory factor (MIF), thymic stromal lymphopoietin (TSLP) and vascular endothelial growth factor (VEGF). The final panel encompassed 17 biomarkers in total, which were selected based on their relevance in studies of cutaneous immunity.
MATERIALS AND METHODS
Skin specimens
Human abdominal skin was obtained from anonymous donors undergoing cosmetic surgery (n = 18) and was used in this study according to the Danish Act on Research Ethics Review of Health Research Projects, Section 14 (3), stating that the use of anonymized human material does not require ethical approval. The skin was prepared immediately upon arrival by mechanical removal of subcutaneous fat, followed by excision of appropriate skin pieces. Areas with stretch marks or other macroscopically visible irregularities were avoided. The skin specimens were frozen at –20°C at least overnight prior to use in the skin reservoir model.
Microdialysis setup
Linear microdialysis probes (EP Ultra High Flux Probes 3,000 kDa) obtained from EP Medical (Copenhagen, Denmark) were used in all microdialysis setups. The probes consisted of hollow semi-permeable 3,000 kDa MWCO membranes glued onto an inlet tubing. The length of the membrane was adjusted to 4 cm and the probe inlet tubing was connected to disposable 1 ml syringes (Plastipak 1 ml Luer, catalogue number (cat. no.) 300013, BD, Madrid, Spain) pre-filled with perfusate, which were mounted in a syringe pump (NE-1200, New Era Pump Systems, Farmingdale, NY, USA). The flow rate was set at 0.8 μl/min, unless otherwise stated. The standard perfusate consisted of human albumin (HA) (20% solution, cat. no. 109697, CSL Behring, Marburg, Germany) diluted to 1% in Ringer-lactate (Fresenius Kabi AB, Uppsala, Sweden) with a final pH of 7.0. For experiments investigating the effect of changing the perfusate composition, 13.4 mM lactic acid (pH 3.9, cat. no. AMPQ40168.1000, Ampliqon, Odense, Denmark) was added to a final concentration of 4 mM lactic acid together with 1% HA in Ringer-lactate, giving this perfusate a pH of 6.0.
The skin reservoir model setup
Frozen skin specimens were allowed to thaw at room temperature before being pinned onto Styrofoam dermis-side down. A moist blotting paper was placed in-between the skin and the foam to keep the skin hydrated from the dermal side during the entire procedure. Microdialysis probes were flushed with Ringer-lactate and primed the perfusate (containing HA) at the same flow rate used for the subsequent experiment for at least 1 h in order to minimize non-specific adsorption of analytes. Primed probes were inserted into thawed skin using 21G guide cannulas (Microlance 3, cat. no. 304431, BD, Fraga, Spain) spanning 2 cm of the skin. The bevel of the guide cannulas was used as a guide to ensure an even insertion depth. Probe functionality was ensured before reference or control solutions were injected by 2×50 μl injections < 1 mm away from each inserted probe on alternating sides and at an even distance along the length of the probe to cover the intradermal span. The injections, which were made in the uppermost dermis, created 2 macroscopically visible skin blebs, which served as reference solution reservoirs. Microdialysis sampling was carried out for 2 h at room temperature with a continuous collection of dialysates in PCR tubes (cat. no. 732-0548, VWR, Radnor, PA, USA) covered with Parafilm to minimize sample evaporation. The entire arrangement was tilted to an angle of approximately 45°, as this was found to improve fluid recovery. All samples were stored at –80°C until analysis.
Fluid recovery
Volume output from the microdialysis probes was monitored by weighing PCR tubes before and after sampling. Fluid recovery was defined as: volumedialysate/volumeexpected × 100%, where volumeexpected=flow rate×sampling time.
Quantification of biomarker concentrations
Biomarker concentrations in dialysates and reference solutions were quantified using commercially available sandwich enzyme-linked immunosorbent assays (ELISA) kits from R&D (Minneapolis, MN, USA): Human CCL27/CTACK DuoSet (cat. no. DY376), Human CXCL1/GROα DuoSet (cat. no. DY275), Human CXCL7/NAP-2 DuoSet (cat. no. DY393), Human CXCL10/IP-10 DuoSet (cat. no. DY266), Human EGF DuoSet (cat. no. DY236), Human GM-CSF DuoSet (cat. no. DY215), Human IFN-γ DuoSet (cat. no. DY285), Human IL-1α DuoSet (cat. no. DY200), Human IL-6 DuoSet (cat. no. DY206), Human IL-8 DuoSet (cat. no. DY208), Human IL-17 DuoSet (cat. no. DY317), Human IL-22 DuoSet (cat. no. DY782), Human IL-23 DuoSet (cat. no. DY1290), Human MIF DuoSet (cat. no. DY289), Human TNF-α DuoSet (cat. no. DY210), Human TSLP DuoSet (cat. no. DY1398) and Human VEGF DuoSet (cat. no. DY293B). All assays were performed according to the manufacturer’s instructions, and optical density was determined at 492 nm with a reference wavelength of 630 nm using a Varioskan Flash Multimode Reader (Thermo Scientific) together with the SkanIt Software (v2.4.5 RE, Thermo Scientific). Lower limit of quantification (LLOQ) was defined as the lowest concentration on the standard curve of respective ELISA.
Background biomarkers levels in fresh and thawed skin
Background tissue levels of IL-1α, IL-6, IL-17 and TNF-α were assessed by microdialysis sampling in fresh and thawed skin (stored overnight at –20°C) from the same donor (D22). Twelve probes were inserted into each skin specimen, followed by 2×50 μl injections of standard perfusate at every probe. Three dialysates from each skin specimen were randomly assigned to quantification of IL-1α, IL-6, IL-17 or TNF-α by ELISA.
Background biomarker levels depending on duration of skin freezing
Background tissue levels of IL-1α, IL-6, IL-17 and TNF-α were measured in thawed skin specimens from 3 donors (D19, D20, and D21) after freezing at –20°C for 1, 7 and 21 days. After thawing, 4 probes were inserted into each skin specimen and 2×50 μl standard perfusate were injected along every probe prior to microdialysis sampling. Equal volumes of 4 replicate dialysates were pooled and concentrations of IL-1α, IL-6, IL-17 and TNF-α were determined by ELISA.
Biomarker reference solutions
Reference solutions of CCL27/CTACK, CXCL1/GROα, CXCL7/NAP-2, CXCL10/IP-10, EGF, GM-CSF, IFN-γ, IL-1α, IL-6, IL-8, IL-17, IL-22, IL-23, MIF, TNF-α, TSLP and VEGF used for injection in the skin reservoir model were prepared from analyte standards provided with the R&D ELISA kits. Titration ranges were made for IL-1α, IL-6, IL-17 and TNF-α by diluting the cytokines in the standard perfusate, either by 3 times serial dilutions of IL-1α (233, 700, and 2,100 pg/ml) or by 2.5 serial dilutions of IL-6 (384, 960, and 2,400 pg/ml), IL-17 (560, 1,400, and 3,500 pg/ml) and TNF-α (561, 1,404, and 3,510 pg/ml). Reference solutions of the remaining biomarkers were made by dissolving the biomarker standard in perfusate to the following concentrations; CCL27/CTACK: 14.25 ng/ml, CXCL1/GROα: 7,200 pg/ml, CXCL7/NAP-2: 4,200 pg/ml, CXCL10/IP-10: 7,700 pg/ml, EGF: 1,200 pg/ml, GM-CSF: 3,625 pg/ml, IFN-γ: 3,900 pg/ml, IL-8: 7,200 pg/ml, IL-22: 8,500 pg/ml, IL-23: 31.2 ng/ml, MIF: 7,700 pg/ml, TSLP: 8,100 pg/ml and VEGF: 7,200 pg/ml.
Titration of the 4 model biomarkers in the skin reservoir model
Reference solutions of IL-1α, IL-6, IL-17 and TNF-α were prepared as described above and injected simultaneously into 4 separate skin specimens from the same donor. Triplicate probes were used for each concentration injected and for the background control (n = 12 probes per skin specimen). The setup was replicated in thawed skin from 3 donors (D4, D18 and D20). Background biomarker levels from control sites were subtracted before the relative skin recoveries were calculated. The relative skin recovery was defined as: concentrationdialysate/concentrationinjected ×100%.
Relative skin recovery of IL-6 depending on flow rate
Four probes were inserted into thawed skin and 2×50 μl IL-6 reference solution (2,400 pg/ml) was injected along 3 probes, while standard perfusate was injected along the last probe serving as a background control. This setup was replicated in 3 skin specimens from the same donor (D7), which were each perfused with a different flow rate: 0.5, 0.8 or 1.5 μl/min. Background biomarker levels from control sites were subtracted before the flow rate-dependent relative skin recovery of IL-6 was calculated.
Relative skin recovery of biomarkers depending on perfusate composition
Relative skin recoveries of the 17 biomarkers were measured in response to probe perfusion with different perfusates: standard perfusate (1% HA in Ringer-lactate, pH 7.0) or perfusate containing lactic acid (1% HA + 4 mM lactic acid in Ringer-lactate, pH 6.0). Six probes were inserted into thawed skin specimens, perfusate (standard or with lactic acid) was injected along half of the probes (serving as control sites) and reference solutions (see concentrations above) were injected along the remaining 6 probes. This setup was repeated for all 17 biomarkers, each tested in skin from a single donor, except for IL-6 and TNF-α, which were assessed in skin from 2 donors (CCL27/CTACK: D41, CXCL1/GROα: D31, CXCL7/NAP-2: D26, CXCL10/IP-10: D32, EGF: D23, GM-CSF: D31, IFN-γ: D11, IL-1α: D5, IL-6: D17 and D26, IL-8: D30, IL-17: D25, IL-22: D11, IL-23: D23, MIF: D1, TNF-α: D17 and D25, TSLP: D23 and VEGF: D32).
Statistical analyses
Statistical analyses were made using GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Outliers were identified using Grubb’s test (the extreme studentized deviate method) based on a 0.05 significance level (2-sided). Values classified as outliers are depicted in graphs (marked ×), but were omitted from statistical analyses, which were performed on the remaining data-sets, including biomarker concentrations below LLOQ. Values below LLOQ are depicted in graphs by open symbols. The statistical tests used are specified in figure legends and corresponding text with asterisks indicating the p-values obtained: *p < 0.05, **p < 0.01.
RESULTS
Fluid recovery from microdialysis probes in the skin reservoir model
Leakage from high MWCO microdialysis probes is a recurrent problem when recovery studies are performed in vitro by sampling through probes submerged in the reference solutions. Therefore, the fluid recovery from 3,000 kDa MWCO microdialysis probes in the skin reservoir model was investigated. It was found to be stable between 80% and 110% of the expected volume output.
Background biomarker levels in fresh and thawed skin
To investigate whether biomarker levels measured in the dialysates were affected by preformed mediators contained in the thawed skin, background tissue levels of the 4 cytokines serving as model analytes (IL-1α, IL-6, IL-17 and TNF-α) were measured by microdialysis. Only IL-1α was detected in dialysate concentrations above LLOQ, whereas neither IL-6, IL-17 nor TNF-α were found in the dialysates. When comparing background tissue levels in thawed skin with background levels in fresh skin from the same donor (Fig. S11), no statistically significant difference was observed (p=0.2162). In order to exclude the duration of skin freezing as a factor potentially affecting background tissue levels, we quantified concentrations of the model biomarkers in thawed skin specimens after storage at –20°C for 1, 7, and 21 days (Fig. S21). Again, IL-1α was the only biomarker detected in concentrations above LLOQ, but the mean background levels of IL-1α were not significantly correlated with the duration of freezing (p = 0.9131, n = 3 donors) (Fig. S21).
Absolute skin recoveries of the model biomarkers in the skin reservoir model
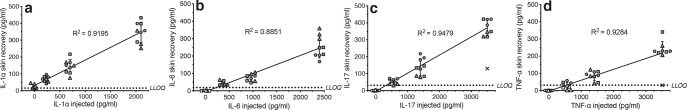
Absolute skin recoveries, defined as the absolute analyte concentration in the dialysates, of the 4 model biomarkers (IL-1α, IL-6, IL-17 and TNF-α) were assessed using the skin reservoir model. A reference solution titration range of each biomarker was injected into thawed skin from 3 donors followed by microdialysis sampling through the skin sites with reference solutions injected. The absolute skin recoveries exhibited a linear correlation with the concentration of the reference solution injected, as all 4 R2 values were > 0.88 (Fig. 2). The mean relative skin recoveries of the model biomarkers are indicated by the slope of the fitted lines, but these values do not account for potential background tissue levels: IL-1α) y=0.1507*x+31.39, IL-6) y=0.1045*x-5.019, IL-17) y=0.1069*x-4.280 and TNF-α) y=0.07013*x-6.589 (y represents the relative skin recovery and x is the concentration of reference solution injected).
Fig. 2.
Absolute skin recovery of model biomarkers. Skin recovery of: (a) interleukin (IL)-1α, (b) IL-6, (c) IL-17, and (d) tumour necrosis factor alpha (TNF-α) measured in the skin reservoir model after injection of reference solutions: IL-1α: 0, 233, 700 and 2,100 pg/ml; IL-6: 0, 384, 960 and 2,400 pg/ml; IL-17: 0, 560, 1400 and 3,500 pg/ml; and TNF-α: 0, 561, 1,404 and 3,510 pg/ml. Dialysate concentrations were determined by enzymelinked immunoassay (ELISA). Data are depicted as individual samples (triplicate probes were used for each biomarker concentration injected and every setup was repeated in skin specimens from 3 donors) with mean ± standard deviation. Symbols represent different skin donors (● D4, ▪ D18, ▴ D20). Open symbols indicate values below the lower limit of quantification (LLOQ), and × denotes outliers, which were omitted from statistical analyses. Linear regression lines are depicted together with the corresponding R2 values.
Relative skin recoveries of the model biomarkers in the skin reservoir model
The relative skin recovery of IL-1α, IL-6, IL-17 and TNF-α was calculated from the concentration of reference solutions injected and the absolute skin recoveries obtained in the skin reservoir model (Fig. 2) after subtraction of background tissue levels (Table I and Fig. 3).
Table I.
Relative skin recovery of model biomarkers
| Biomarker | Concentration injected (pg/ml)a | Relative skin recovery (%)b |
|
|---|---|---|---|
| Mean ± SD | CV | ||
| IL-1α |
|
15.2 ± 5.1 |
33.7 |
| 233 | 15.7 ± 6.7 | 42.9 | |
| 700 | 13.9 ± 5.2 | 37.2 | |
| 2,100 |
16.2 ± 3.3 |
20.3 |
|
| IL-6 |
|
9.3 ± 2.9 |
30.9 |
| 384 | 9.9 ± 3.1 | 31.3 | |
| 960 | 7.3 ± 1.9 | 25.9 | |
|
|
2,400 |
10.6 ± 2.5 |
23.8 |
| IL-17 |
|
10.7 ± 2.6 |
24.9 |
| 560 | 10.5 ± 3.3 | 31.3 | |
| 1,400 | 10.6 ± 3.2 | 30.1 | |
|
|
3,500 |
10.9 ± 2.6 |
22.4 |
| TNF-α |
|
6.5 ± 2.7 |
40.9 |
| 561 | 5.4 ± 3.5 | 66.0 | |
| 1,404 | 6.4 ± 2.0 | 31.6 | |
| 3,510 | 7.9 ± 1.6 | 19.7 | |
Reference solutions of interleukin (IL)-1α, IL-6, IL-17 and tumour necrosis factor (TNF)-α injected into thawed skin specimens before sampling.
Mean relative skin recovery is based on data obtained from triplicate probes, which were used for every biomarker concentration and repeated in skin specimens from 3 donors (D4, D18, and D20).
SD: standard deviation; CV: coefficient of variation.
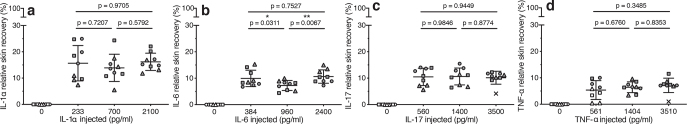
Fig. 3.
Relative skin recovery of model biomarkers. The relative skin recovery of: (a) interleukin (IL)-1α, (b) IL-6, (c) IL-17 and (d) tumour necrosis factor (TNF)-α calculated from the absolute skin recovery of the biomarkers with background tissue levels subtracted and the concentration of the corresponding reference solutions injected: IL-1α: 0, 233, 700 and 2,100 pg/ml; IL-6: 0, 384, 960 and 2,400 pg/ml; IL-17: 0, 560, 1,400 and 3,500 pg/ml; and TNF-α: 0, 561, 1,404 and 3,510 pg/ml. Dialysate concentrations were determined by enzyme-linked immunoassay (ELISA). Data are depicted as individual samples (triplicate probes for each biomarker concentration injected and every setup was repeated in skin specimens from 3 donors) with mean ± standard deviation. Symbols represent different skin donors (● D4, ▪ D18, ▴ D20). Open symbols indicate values below the lower limit of quantification (LLOQ) and × denotes outliers, which were omitted from statistical analyses. p-values are obtained from a 2-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test comparing the mean relative skin recoveries after injection of different reference solutions.
Mean relative skin recoveries of the 4 cytokines ranged between 6.5% and 15.2% (Table I and Fig. 3), with IL-1α having the highest mean relative skin recovery: 15.2% (Fig. 3a), followed by IL-17: 10.7% (Fig. 3c), IL-6: 9.3% (Fig. 3b), and TNF-α: 6.5% (Fig. 3d). A 2-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to assess whether skin donor and the periprobe biomarker concentration were significant factors influencing the relative skin recoveries. The current study found that only the relative skin recovery of IL-6 was significantly correlated with the concentration of the reference solution injected (p = 0.0061), whereas the relative skin recovery of IL-1α did not depend on the concentration injected (p = 0.5802), nor did the relative skin recovery of IL-17 (p = 0.8802) or TNF-α (p = 0.1234). When investigating the possible effect of skin donor on the relative skin recoveries obtained, this factor was found to be statistically significant for IL-6 (p = 0.0172) and IL-17 (p = 0.0003), but not for IL-1α (p = 0.0548) or TNF-α (p = 0.3188) (n = 3 donors).
Relative skin recovery of IL-6 depending onflow rate
It is well-established that the relative recovery in vitro is inversely correlated with flow rate (2,14,16) and the current study therefore assessed this in the skin reservoir model using IL-6 as a model biomarker. When measuring the relative skin recovery of IL-6 at 3 different flow rates an inverse relationship between these 2 variables in the skin reservoir model was observed, as depicted in Fig. S31. The non-linear fit had a R2-value of 0.9712.
Relative skin recoveries in response to different perfusate compositions
Encouraged by successful recovery of the 4 model biomarkers IL-1α, IL-6, IL-17 and TNF-α in the skin reservoir model, we decided to expand the biomarker panel. The relative skin recoveries of 13 additional biomarkers (CCL27/CTACK, CXCL1/GROα, CXCL7/NAP-2, CXCL10/IP-10, EGF, GM-CSF, IFN-γ, IL-8, IL-22, IL-23, MIF, TSLP and VEGF) were assessed, together with the effect of changing the composition of the perfusate.
The perfusate pH was modified by adding lactic acid to a final concentration of 4 mM, which corresponds to lactate levels at the onset of lactate accumulation in the blood (17), and relative skin recoveries were obtained for all 17 biomarkers using both perfusates (Fig. S41 and Table II). Only the relative skin recovery of EGF was significantly affected by the addition of lactic acid (Fig. S41 and Table II), as the relative skin recovery decreased significantly with the acidic perfusate compared with skin perfusion with the standard composition (9.8% vs. 12.7%, p = 0.007). Furthermore, it was found that IFN-γ could not be recovered using the 3,000 kDa probes regardless of the perfusate composition, being the only biomarker out of the 17 investigated (Fig. S41 and Table II). When assessing the relative skin recovery of CCL27/CTACK and MIF, background tissue levels of the 2 biomarkers were found to exceed the concentration of reference solution injected, thus it was not possible to estimate the relative skin recovery of CCL27/CTACK and MIF in the skin reservoir model. However, the high dialysate levels clearly demonstrated that the 2 biomarkers were able to pass the microdialysis membrane. Mean relative skin recoveries of the remaining biomarkers were as follows (standard perfusate/perfusate with 4 mM lactic acid): CXCL1/GROα (7.9%/7.4%), CXCL7/NAP-2 (5.7%/9.7%), CXCL10/IP-10 (0.6%/0.9%), GM-CSF (13.3%/12.4%), IL-1α (14.6%/12.2%), IL-6 (8.9%/11.4%), IL-8 (18.4%/16.7%), IL-17 (11.2%/8.7%), IL-22 (7.7%/11.4%), IL-23 (5.2%/4.8%), TNF-α (7.5%/10.1%), TSLP (4.0%/4.3%) and VEGF (4.8%/3.8%). Apart from EGF, none of the biomarkers were significantly affected by lowering the pH of the perfusate (Fig. S41 and Table II).
Table II.
Relative skin recovery depending on perfusate compositiona
| Biomarker | Perfusate compositionb | Relative skin recovery (%) |
p-valuec | n (skin donor identifier)d | |
|---|---|---|---|---|---|
| Mean ± SD | CV | ||||
| CCL27/CTACK | Standard | n/a | n/a | n/a | 1 (D41) |
| CCL27/CTACK | Standard | n/a | n/a | n/a | 1 (D41) |
| + lactic acid | n/a | n/a | |||
| CXCL1/GROα | Standard | 7.9 ± 1.0 | 12.5 | 0.6348 | 1 (D31) |
| + lactic acid | 7.4 ± 1.1 | 14.8 | |||
| CXCL7/NAP-2 | Standard | 5.7 ± 0.4 | 7.9 | 0.0889 | 1 (D26) |
| + lactic acid | 9.7 ± 2.3 | 23.5 | |||
| CXCL10/IP-10 | Standard | 0.6 ± 0.2 | 29.2 | 0.2647 | 1 (D32) |
| + lactic acid | 0.9 ± 0.3 | 30.6 | |||
| EGF | Standard | 12.7 ± 0.5 | 4.2 | 0.0044 | 1 (D23) |
| + lactic acid | 9.8 ± 0.2 | 1.7 | |||
| GM-CSF | Standard | 13.3 ± 2.0 | 15.1 | 0.4953 | 1 (D31) |
| + lactic acid | 12.4 ± 0.5 | 3.7 | |||
| IFN-γ | Standard | 0.0 ± n/a | n/a | n/a | 1 (D11) |
| + lactic acid | 0.0 ± n/a | n/a | |||
| IL-1α | Standard | 14.6 ± 2.5 | 16.8 | 0.2219 | 1 (D5) |
| + lactic acid | 12.2 ± 0.9 | 7.5 | |||
| IL-6 | Standard | 8.9 ± 2.3 | 25.8 | 0.0699 | 2 (D17/D26) |
| + lactic acid | 11.4 ± 1.9 | 16.4 | |||
| IL-8 | Standard | 18.4 ± 1.4 | 7.7 | 0.2912 | 1 (D30) |
| + lactic acid | 16.7 ± 2.0 | 11.9 | |||
| IL-17 | Standard | 11.2 ± 0.6 | 5.1 | 0.0693 | 1 (D25) |
| + lactic acid | 8.7 ± 1.3 | 15.1 | |||
| IL-22 | Standard | 7.7 ± 0.8 | 10.4 | 0.1030 | 1 (D11) |
| + lactic acid | 11.4 ± 2.3 | 20.4 | |||
| IL-23 | Standard | 5.2 ± 0.8 | 14.4 | 0.8047 | 1 (D23) |
| + lactic acid | 4.8 ± 2.6 | 54.3 | |||
| MIF | Standard | n/a | n/a | n/a | 1 (D1) |
| + lactic acid | n/a | n/a | |||
| TNF-α | Standard | 7.5 ± 1.9 | 25.4 | 0.0555 | 2 (D17/D25) |
| + lactic acid | 10.1 ± 2.1 | 20.5 | |||
| TSLP | Standard | 4.0 ± 0.9 | 23.7 | 0.6458 | 1 (D23) |
| + lactic acid | 4.3 ± 0.7 | 16.8 | |||
| VEGF | Standard | 4.8 ± 1.1 | 22.3 | 0.2679 | 1 (D32) |
| + lactic acid | 3.8 ± 0.7 | 19.1 | |||
Data are depicted in Fig. S41.
Relative skin recoveries obtained in response to perfusion with standard perfusate or perfusate supplemented with lactic acid to a final concentration of 4 mM.
p-values are obtained using t-tests with Welch’s correction. n/a=not applicable.
The perfusate-dependent relative skin recovery of each biomarker was investigated in skin from 1 donor, except for IL-6 and TNF-α, which were assessed in skin from 2 donors. Significant value is given in bold.
SD: standard deviation; CV: coefficient of variation.
DISCUSSION
In 2007, the US Food and Drug Administration and the American Association of Pharmaceutical Scientists published a white paper encouraging more widespread use of microdialysis in the preclinical- and clinical phases of pharmacological studies, leading to an increased interest in skin microdialysis and its possible applications (1, 18). The technique is versatile and serves as a minimally invasive alternative to biopsies and skin suction blisters, e.g. in studies of drug delivery, pharmacokinetics/pharmacodynamics, drug metabolism or cutaneous immunity in general (3). It is, however, important to recognize and acknowledge the technical difficulties and potential pitfalls associated with microdialysis sampling of larger molecules, and these must be addressed before in vivo microdialysis experiments are initiated (2, 10, 18). Preliminary feasibility studies are thus essential to evaluate whether the technique facilitates successful recovery of the molecule(s) of interest, but, despite attempts from more groups (11, 12), there are currently no standardized in vitro systems available for this purpose. Therefore, we set out to develop such a validation tool.
The model presented in this paper uses previously frozen human ex vivo skin as a reference solution reservoir; hence it was named “the skin reservoir model”. Biomarker recovery was estimated by sampling through reference solutions contained in the inert skin matrix and different parameters were adjusted to study the effect on sampling of the 17 large biomarkers, which were chosen due to their relevance in dermal processes, such as wound healing and inflammatory skin conditions, including psoriasis and atopic dermatitis (19–24). Other groups have used microdialysis in combination with thawed skin specimens to investigate absorption of topically applied drugs (25–27), but thawed skin has (to the best of our knowledge) never been used to evaluate sampling feasibility by skin microdialysis.
Due to reports of ultrafiltration and lack of fluid recovery when sampling through high MWCO probes in vitro (8, 10–12, 14, 15, 28), the main goal of this study was to establish a setup not afflicted by these issues and thus enabling validation of biomarker recovery across high MWCO membranes. It was found that sampling through 3,000 kDa MWCO probes in the skin reservoir model was not associated with probe leakage, which may (in part) be attributed to a pressure exerted on the probe by the structural matrix of the skin, as opposed to in vitro sampling through reference solutions contained in a tube. In addition, colloid additives (here in the form of 1% HA) may counteract the osmotic imbalances described by other groups (10, 12). The concentration of HA in the perfusate was set based on previous experience combined with reports from the scientific literature (29). Apart from affecting the colloid osmotic pressure, albumin has the additional advantage of acting as a carrier protein, thereby stabilizing potentially volatile biomarkers. Furthermore, HA decreases non-specific adsorption of molecules to probe materials (e.g. membrane or inlet tubing) and therefore serves to enhance recovery and stability of the analytes in the dialysates (8, 11, 14, 30).
It is well known that preformed mediators can be released upon skin trauma, e.g. from probe implementation, or due to freeze/thaw-mediated cell lysis (23, 31, 32). In line with this, high background levels of CCL27/CTACK and MIF were found in thawed skin, whereas background tissue levels of the remaining 15 biomarkers were below LLOQ or detected only in very low concentrations, making it easy to account for by subtracting background levels before calculating the relative skin recoveries.
The human skin specimens can be stored for longer periods at –20°C prior to use, as no significant change was observed in background tissue levels in response to the duration of skin freezing. This is in line with other studies stating that skin stored at –20°C maintains its barrier integrity, and storage at this temperature is less damaging compared with freezing at –80°C (26, 33). Thus, thawed skin serves as a suitable reservoir for reference solutions if the analyte of interest is not already found in the tissue at high concentrations, as this hampers assessment of the relative skin recovery.
The relative skin recovery must be independent of the analyte concentration in the periprobe environment in order for the skin reservoir model to be a reliable tool for estimating biomarker recoveries. This entails that the absolute recovery of the analyte must be linearly correlated with the analyte concentration in the reference solution injected (11, 18, 34, 35), which was demonstrated for the 4 model biomarkers (IL-1α, IL-6, IL-17, and TNF-α). The linear correlation indicates that there are only minor interactions between analytes, probe components and the surrounding milieu, which may otherwise impede a fair estimation of analyte recovery (11, 36).
For 2 of the model biomarkers, IL-6 and IL-17, the skin donor was a significant factor on biomarker recovery; however, we believe that this is due to the low number of donors and replicate probes included in this study. Furthermore, the relative skin recovery of IL-6 was significantly affected by the concentration in the periprobe fluid, although this is also attributed to the limited number of skin donors available. Slight differences in membrane construction, injection of reference solutions, intradermal probe placement and variations in skin thickness, lipid content, moisture level and elasticity between donors may account for the interprobe variations observed in fluid- and analyte recovery. One of these factors, the intradermal probe depth, can be assessed in future studies using a 20 MHz ultrasound scanner (25, 37). Overall, the variability observed in our study underlines the need for technically repeated measurements in the form of multiple probes measuring identical conditions in the same donor skin.
In line with the theoretical principle of microdialysis sampling described (2, 16, 35), the relative recovery of IL-6 was found to be inversely correlated with the flow rate in the skin reservoir model. The choice of flow rate is a compromise between sample volume, sampling time and analyte recovery. Since large biomarkers, such as cytokines and growth factors, are often short-lived molecules (38), it is advisable to keep the sampling intervals as short as possible to avoid analyte breakdown in the dialysates and to ensure a good temporal resolution. However, an adequate volume must be obtained for subsequent analyses, which in our case was carried out using volume-consuming ELISA (1), and the flow rate was therefore set at 0.8 μl/min as a compromise between the above-mentioned factors.
Perfusate composition is another factor that might affect analyte recovery (2, 11, 34). Kirbs & Kloft (11) found that the relative recovery of some analytes is affected by pH, which incited us to test the influence of perfusate pH in the skin reservoir model, as changing the pH of the perfusate may be an easy way of optimizing a microdialysis setup to enhance analyte recovery. We hypothesized that a local change in pH might affect molecular interactions between the analyte and components of the extracellular matrix. When this was assessed in the skin reservoir model, we found that the relative skin recovery of one biomarker, EGF, was significantly reduced upon addition of lactic acid to the perfusate, whereas the other 16 biomarkers were not significantly affected. This illustrates that the skin reservoir model also facilitates optimization of experimental setups, as different parameters potentially influencing biomarker recovery can be investigated and adjusted in order to obtain the highest possible recovery of the target biomarker(s). However, a limitation of the model is the lack of blood flow, which has been shown to affect the relative recovery in vivo (39), which could cause overestimation of the relative skin recovery using this ex vivo model compared with the actual relative recovery obtainable in vivo.
Holmgaard et al. suggest a threshold of 4–5% relative recovery in vitro to indicate if a given biomarker is likely to be recovered in vivo (18). When applying this cut-off to the data obtained from the skin reservoir model, 13 of the 17 biomarkers were above the threshold. Although high background levels of CCL27/CTACK and MIF in thawed skin hampered quantification of the exact relative skin recovery, we expect these biomarkers to be recoverable from viable skin, as the high concentration of CCL27/CTACK and MIF in the dialysates indicated passage of the biomarkers across the microdialysis membrane. IFN-γ was the only biomarker not recovered using the skin reservoir model; however, this is consistent with the low in vitro recoveries reported for IFN-γ (6, 29, 40). Whether an analyte can be detected in dialysates from human skin will ultimately depend on a combination of the tissue concentration, the relative analyte recovery in the given setup, and the sensitivity of the analysis platform used (34).
As tempting as it is to compare the relative skin recoveries obtained in the skin reservoir model with analyte recoveries in previous in vitro studies, differences in the experimental setups, such as probe material, MWCO of the membrane, perfusate composition and flow rate render direct comparisons across studies difficult. In addition, in contrast to previous in vitro approaches, the skin reservoir model allows for potential interactions between analytes and the extracellular matrix, which might also influence microdialysis recovery of biomarkers from skin in vivo (6). This added complexity is an advantage of the skin reservoir model, as it more closely resembles the cutaneous environment in which in vivo sampling takes place. Accordingly, Shukla et al. (2) recommended that estimations of relative recoveries were performed in a matrix mimicking the target tissue of subsequent investigations; in this case the skin.
In summary, this paper presents a new approach for assessing the applicability of using microdialysis to sample large biomarkers from human skin.
ACKNOWLEDGEMENTS
The authors thank Hanna Smith Clementsen for helpful comments and proofreading of the manuscript.
Conflicts of interest. KB is employed as Industrial PhD student by RefLab ApS. NPHK is employed as Scientist by RefLab ApS. sF is a former employee at RefLab ApS. PSS is acting as research consultant for RefLab ApS and EP Medical. The other authors have no competing interests to declare.
REFERENCES
- 1.Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res 2007; 24: 1014–1025. [DOI] [PubMed] [Google Scholar]
- 2.Shukla C, Bashaw ED, Stagni G, Benfeldt E. Applications of dermal microdialysis: a review. J Drug Deliv Sci Technol 2014; 24: 259–269. [Google Scholar]
- 3.Baumann KY, Church MK, Clough GF, Quist SR, Schmelz M, Skov PS, et al. Skin microdialysis: methods, applications and future opportunities – an EAACI position paper. Clin Transl Allergy 2019; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen LJ, Brasso K, Pryds M, Skov PS. Histamine release in intact human skin by monocyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1α, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. J Allergy Clin Immunol 1996; 98: 790–796. [DOI] [PubMed] [Google Scholar]
- 5.Petersen LJ, Church MK, Skov PS. Histamine is released in the wheal but not the flare following challenge of human skin in vivo: a microdialysis study. Clin Exp Allergy 1997; 27: 284–295. [DOI] [PubMed] [Google Scholar]
- 6.Ao X, Stenken JA. Microdialysis sampling of cytokines. Methods 2006; 38: 331–341. [DOI] [PubMed] [Google Scholar]
- 7.Jadhav SB, Khaowroongrueng V, Derendorf H. Microdialysis of large molecules. J Pharm Sci 2016; 105: 3233–3242. [DOI] [PubMed] [Google Scholar]
- 8.Clough GF. Microdialysis of large molecules. AAPS J 2005; 7: E686–E692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hersini KJ, Melgaard L, Gazerani P, Petersen LJ. Microdialysis of inflammatory mediators in the skin: A review. Acta Derm Venereol 2014; 94: 501–511. [DOI] [PubMed] [Google Scholar]
- 10.Helmy A, Carpenter KLH, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma 2009; 26: 549–561. [DOI] [PubMed] [Google Scholar]
- 11.Kirbs C, Kloft C. In vitro microdialysis recovery and delivery investigation of cytokines as prerequisite for potential biomarker profiling. Eur J Pharm Sci 2014; 57: 48–59. [DOI] [PubMed] [Google Scholar]
- 12.Khan F, Pharo A, Lindstad JK, Mollnes TE, Tønnessen TI, Pischke SE. Effect of perfusion fluids on recovery of inflammatory mediators in microdialysis. Scand J Immunol 2015; 82: 467–475. [DOI] [PubMed] [Google Scholar]
- 13.Duo J, Stenken J a. In vitro and in vivo affinity microdialysis sampling of cytokines using heparin-immobilized microspheres. Anal Bioanal Chem 2011; 399: 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trickler WJ, Miller DW. Use of osmotic agents in microdialysis studies to improve the recovery of macromolecules. J Pharm Sci 2003; 92: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 15.Chu J, Koudriavtsev V, Hjort K, Dahlin AP. Fluorescence imaging of macromolecule transport in high molecular weight cut-off microdialysis. Anal Bioanal Chem 2014; 406: 7601–7609. [DOI] [PubMed] [Google Scholar]
- 16.Wælgaard L, Pharo A, Tønnessen TI, Mollnes TE. Microdialysis for monitoring inflammation: efficient recovery of cytokines and anaphylotoxins provided optimal catheter pore size and fluid velocity conditions. Scand J Immunol 2006; 64: 345–352. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin ML, Harris JE, Hernández A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 2007; 1: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol 2010; 23: 225–243. [DOI] [PubMed] [Google Scholar]
- 19.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci 2013; 72: 206–217. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care 2016; 5: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Cerdeira C, Molares-Vila A, Sánchez-Blanco E, Sánchez-Blanco B. Study on certain biomarkers of inflammation in psoriasis through “OMICS” platforms. Open Biochem J 2014; 8: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine 2015; 73: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjögren F, Davidsson K, Sjöström M, Anderson CD. Cutaneous microdialysis: cytokine evidence for altered innate reactivity in the skin of psoriasis patients? AAPS J 2012; 14: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quist SR, Wiswedel I, Quist J, Gollnick HP. Kinetic profile of inflammation markers in human skin in vivo following exposure to ultraviolet B indicates synchronic release of cytokines and prostanoids. Acta Derm Venereol 2016; 96: 910–916. [DOI] [PubMed] [Google Scholar]
- 25.Holmgaard R, Benfeldt E, Bangsgaard N, Sorensen JA, Brosen K, Nielsen F, et al. Probe depth matters in dermal microdialysis sampling of benzoic acid after topical application: an ex vivo study in human skin. Skin Pharmacol Physiol 2012; 25: 9–16. [DOI] [PubMed] [Google Scholar]
- 26.Holmgaard R, Benfeldt E, Nielsen JB, Gatschelhofer C, Sorensen JA, Höfferer C, et al. Comparison of open-flow microperfusion and microdialysis methodologies when sampling topically applied fentanyl and benzoic acid in human dermis ex vivo. Pharm Res 2012; 29: 1808–1820. [DOI] [PubMed] [Google Scholar]
- 27.Döge N, Hönzke S, Schumacher F, Balzus B, Colombo M, Hadam S, et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin – assessment by intradermal microdialysis and extraction from the different skin layers. J Control Release 2016; 242: 25–34. [DOI] [PubMed] [Google Scholar]
- 28.Sides CR, Stenken JA. Microdialysis sampling techniques applied to studies of the foreign body reaction. Eur J Pharm Sci 2014; 57: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain 2008; 139: 15–27. [DOI] [PubMed] [Google Scholar]
- 30.Keeler GD, Durdik JM, Stenken JA. Comparison of microdialysis sampling perfusion fluid components on the foreign body reaction in rat subcutaneous tissue. Eur J Pharm Sci 2014; 57: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenken JA, Church MK, Gill CA, Clough GF. How minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studies. AAPS J 2010; 12: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjögren F, Anderson C. Sterile trauma to normal human dermis invariably induces IL1beta, IL6 and IL8 in an innate response to “danger”. Acta Derm Venereol 2009; 89: 459–465. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen JB, Plasencia I, Sørensen JA, Bagatolli LA. Storage conditions of skin affect tissue structure and subsequent in vitro percutaneous penetration. Skin Pharmacol Physiol 2011; 24: 93–102. [DOI] [PubMed] [Google Scholar]
- 34.Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev 2002; 54: S99–S121. [DOI] [PubMed] [Google Scholar]
- 35.de Lange ECM. Recovery and calibration techniques: toward quantitative microdialysis. In: Müller M, editor. Microdialysis in drug development. Springer, New York, NY, 2013: p. 13–33. [Google Scholar]
- 36.Lindberger M, Tomson T, Lars S. Microdialysis sampling of carbamazepine, phenytoin and phenobarbital in dubcutaneous extracellular fluid and subdural cerebrospinal fluid in humans: an in vitro and in vivo study of adsorption to the sampling device. Pharmacol Toxicol 2002; 91: 158–165. [DOI] [PubMed] [Google Scholar]
- 37.Benfeldt E, Hansen SH, Vølund A, Menné T, Shah VP. Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol 2007; 127: 170–178. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 2010; 13: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clough GF, Boutsiouki P, Church MK, Michel CC. Effects of blood flow on the in vivo recovery of a small diffusible molecule by microdialysis in human skin. J Pharmacol Exp Ther 2002; 302: 681–686. [DOI] [PubMed] [Google Scholar]
- 40.Ao X, Sellati TJ, Stenken JA. Enhanced microdialysis relative recovery of inflammatory cytokines using antibody-coated microspheres analysed by flow cytometry. Anal Chem 2004; 76: 3777–3784. [DOI] [PubMed] [Google Scholar]