Figure 2.
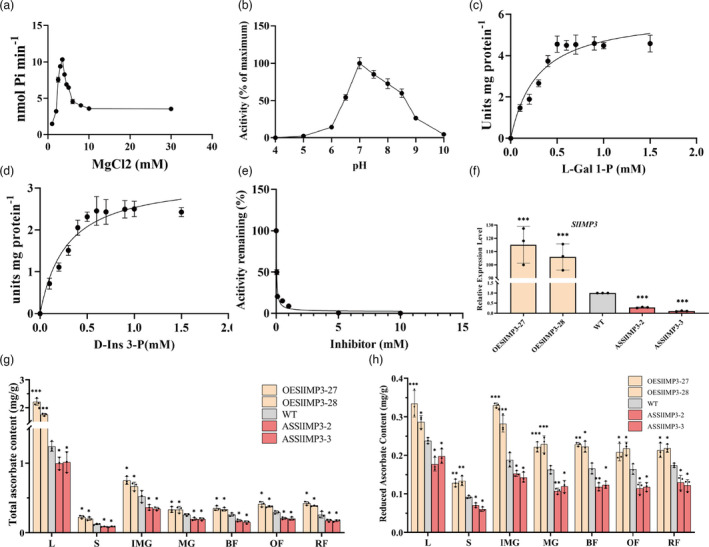
SlIMP3 activity analysis and positive regulation of AsA biosynthesis. (a) Enzyme activity at different magnesium concentrations. A 50 μL of mixture with 50 mm of Tris‐Cl, pH 7.0, 0.5 mm of D‐Ins 3‐P, 2 mg of purified SlIMP3 proteins, and varying concentrations of MgCl2 was used as standard conditions. (b) Enzyme activity at different pH values. Except for the different pH value and the determined 3.5 mm of MgCl2, the other conditions are the same as (a). (c, d) Substrate saturation curve of SlIMP3 at different L‐Gal 1‐P (a) and D‐Ins 3‐P (b) concentrations. Km and Vmax were calculated according to Michaelis–Menten equation by GraphPad Prism 8 software. (e) Inhibition of SlIMP3 activity by LiCl. IC50 were calculated according to substrate inhibition equation by GraphPad Prism 8 software. (f) Relative expression level of SlIMP3 in overexpression (OESlIMP3‐27, OESlIMP3‐28) and antisense (ASSlIMP3‐2, ASSlIMP3‐3) lines. (g, h) Total ascorbate and reduced ascorbate contents in different tomato tissues (L leaf, S stem, IMG immature green fruit, MG mature green fruit, BF break fruit, OF orange fruit, RF red fruit) of WT, OESlIMP3, and ASSlIMP3 lines. All data contain three replicates and SD was given by error bar. Statistical significance between WT and OESlIMP3/ASSlIMP3 lines was indicated by asterisk (*P < 0.05, **P < 0.01, ***P < 0.001).
