Abstract
Introduction
Inhaled gene therapy of muco-obstructive lung diseases requires a strategy to achieve therapeutically relevant gene transfer to airway epithelium covered by particularly dehydrated and condensed mucus gel layer. Here, we introduce a synthetic DNA-loaded mucus-penetrating particle (DNA-MPP) capable of providing safe, widespread and robust transgene expression in in vivo and in vitro models of muco-obstructive lung diseases.
Methods
We investigated the ability of DNA-MPP to mediate reporter and/or therapeutic transgene expression in lung airways of a transgenic mouse model of muco-obstructive lung diseases (ie, Scnn1b-Tg) and in air–liguid interface cultures of primary human bronchial epithelial cells harvested from an individual with cystic fibrosis. A plasmid designed to silence epithelial sodium channel (ENaC) hyperactivity, which causes airway surface dehydration and mucus stasis, was intratracheally administered via DNA-MPP to evaluate therapeutic effects in vivo with or without pretreatment with hypertonic saline, a clinically used mucus-rehydrating agent.
Results
DNA-MPP exhibited marked greater reporter transgene expression compared with a mucus-impermeable formulation in in vivo and in vitro models of muco-obstructive lung diseases. DNA-MPP carrying ENaC-silencing plasmids provided efficient downregulation of ENaC and reduction of mucus burden in the lungs of Scnn1b-Tg mice, and synergistic impacts on both gene transfer efficacy and therapeutic effects were achieved when DNA-MPP was adjuvanted with hypertonic saline.
Discussion
DNA-MPP constitutes one of the rare gene delivery systems providing therapeutically meaningful gene transfer efficacy in highly relevant in vivo and in vitro models of muco-obstructive lung diseases due to its unique ability to efficiently penetrate airway mucus.
INTRODUCTION
Current therapeutic regimens for muco-obstructive lung diseases, including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD), remain largely symptomatic except for a few small molecule-based drugs applicable to genetically defined subpopulations of CF patients. Revelation of numerous genetic targets has dubbed gene therapy as a potential breakthrough to revert natural histories of these notoriously refractory diseases.1 However, clinical trials of inhaled gene therapy to date have failed to demonstrate meaningful benefits largely due to inability to achieve therapeutically relevant gene transfer efficacy in the lung airways.1 2 In particular, inhaled foreign matters, including gene vectors, are readily trapped by protective mucus gel layer covering the airway epithelium and subsequently removed from the lung via mucociliary clearance (MCC) or expectoration prior to reaching the underlying cells.1 3–7 Of note, we and others have confirmed that leading virus-based8 9 and synthetic10–12 gene vectors, including those that have been tested in clinics, cannot efficiently penetrate human pathological airway mucus (ie, CF sputum).
This reality has led to efforts to understand and overcome the challenging barrier posed by airway mucus. Indeed, we found that the airway mucus is a ‘sticky net’, which traps particulate matters via adhesive interactions and steric obstruction.1 3 6 13 Based on these findings, we engineered nanoparticles possessing small diameters, non-adhesive surfaces and physiological stability, which could rapidly penetrate through airway mucus.4 10 12–18 Specifically, we formulated DNA-loaded mucus-penetrating particles (DNA-MPP) using a blend of cationic polymers and polyethylene glycol (PEG) conjugated cationic polymers and confirmed their ability to efficiently penetrate unperturbed sputum samples collected from patients with CF.10 12 19 We also showed that inhalation of DNA-MPP provided widespread airway distribution, prolonged lung retention and/or highly efficient reporter transgene expression in the lungs of healthy inbred mice.10 12
As a next step towards development of DNA-MPP, we sought to extend proof-of-principle studies and establish clinical relevance in an animal model characterised by obstructive airway diseases with mucus stasis. To this end, we evaluated DNA-MPP delivery and efficacy on mice with airway-targeted overexpression of the epithelial sodium channel (ENaC) β subunit (encoded by the Scnn1b gene), one of the few rodent models that recapitulate key features of muco-obstructive lung diseases.1 20 Similar to the affected patients,21 Scnn1b-Tg mice exhibit airway sodium hyperabsorption and surface dehydration, which impairs the MCC, promoting mucus plugging and chronic inflammation.20 22 We also tested whether administration of hypertonic saline, a widely used treatment to acutely promote airway mucus clearance by rehydrating the airway surface, would further increase gene transfer efficacy of DNA-MPP in Scnn1b-Tg mice. Finally, we tested the hypothesis that downregulation of ENaC via inhaled DNA-MPP carrying plasmids encoding short hairpin RNA (shRNA) against ENaC would ameliorate the pathology associated with ENaC overexpression in the lungs of Scnn1b-Tg mice.
RESULTS
We engineered a DNA-MPP platform for inhaled gene therapy of muco-obstructive lung diseases, using a method that we had previously established.10 Specifically, a mixture of poly(β-amino ester) (PBAE) and PEG-conjugated PBAE polymers at an optimised ratio was used to compact plasmids encoding various reporter or therapeutic nucleic acids, including luciferase (~5 kb), green fluorescent protein (GFP ~4.5 kb) or shRNA against ENaC (shENaC, ~6.5 kb). In parallel, we formulated mucus-impermeable DNA-loaded conventional particles (DNA-CP) using PBAE polymers only. We first confirmed that all the DNA-MPP formulations exhibited mucus-penetrating properties, including small particle diameters (~50 nm) and near neutral surface charges regardless of the type/size of plasmid payloads (online supplemental table 1; data represent physicochemical properties of DNA-loaded nanoparticles carrying luciferase-encoding, GFP-encoding and shENaC-encoding plasmids).10 In comparison, DNA-CP possessed larger particle sizes (> 100 nm in diameters) and highly cationic surfaces (ζ-potentials of ~30 mV) (online supplemental table 1). We also confirmed that spraying DNA-CP or DNA-MPP through an aerosol-generating microsprayer does not affect their physicochemical properties (online supplemental table 2). We then conducted gel electrophoretic retardation assay where we found that all three plasmids were stably packaged into DNA-MPP, as evidenced by their complete retention within the wells of the gel (figure 1A). Moreover, DNA-MPP were capable of protecting plasmid payloads from enzymatic degradation by DNase, while free plasmids were completely degraded (figure 1B). We next confirmed that DNA-MPP retained their small particle diameters (~50 nm) in bronchoalveolar lavage (BAL) fluid at 37°C at least up to 4 hours (figure 1C), indicating excellent colloidal stability in a physiologically relevant lung environment. In contrast, DNA-CP immediately aggregated as soon as the incubation commenced, reaching microns of sizes within an hour (figure 1C). Although we have previously demonstrated the ability of DNA-MPP to resist adsorption of lung-derived protein to the particle surface,10 we have not determined binding of mucin to the surface of DNA-MPP. We thus conducted surface plasmon resonance binding assay using a custom-made porcine gastric mucin-immobilised chip to confirm whether DNA-MPP due to the non-adhesive PEG coating, precluded adhesive interactions with mucin. While DNA-CP readily adhered to the mucin chip over time, adhesive interactions between DNA-MPP and the chip were negligible over the identical time period (figure 1D).
Figure 1.
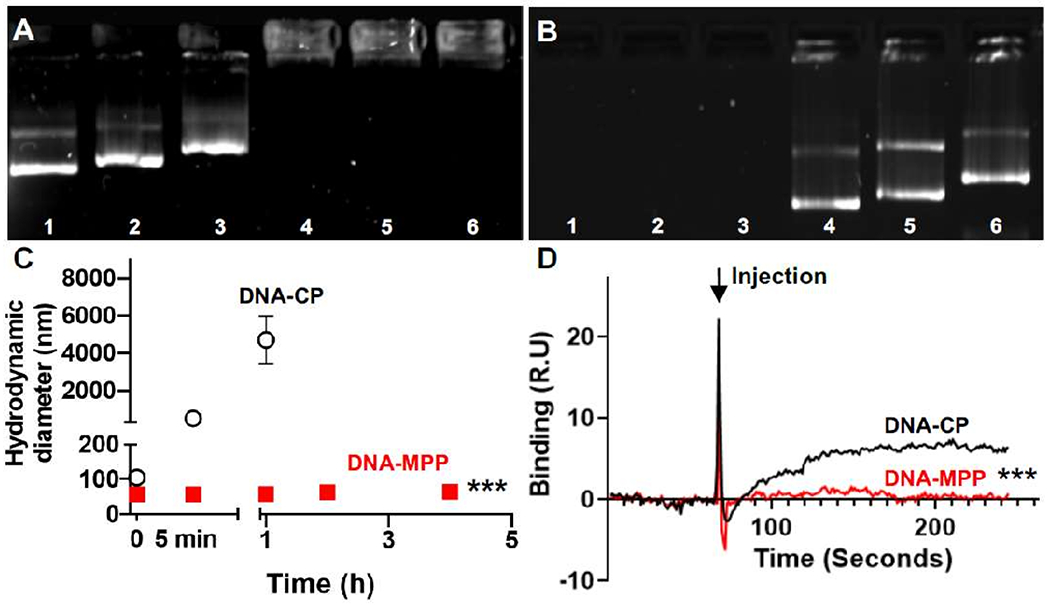
In vitro characterisation of DNA-loaded nanoparticles. Electrophoretic analysis of (A) complexation and (B) protection (against enzymatic degradation by DNase) of plasmid payloads. For the protection assay, plasmids were de-compacted from DNA-MPP via heparin prior to gel electrophoresis. Lane 1: GFP-encoding plasmid. Lane 2: luciferase-encoding plasmid. Lane 3: shENaC-encoding plasmid. Lane 4: MPP carrying GFP-encoding plasmid. Lane 5: MPP carrying luciferase-expressing plasmid. Lane 6: MPP carrying shENaC-encoding plasmid. (C) Colloidal stability of DNA-CP and DNA-MPP in BAL fluid (ie, change in hydrodynamic diameter) over time at 37°C (n=3, mean±SD). ***p<0.001 (Student’s t-test). (D) Binding of DNA-CP and DNA-MPP to porcine gastric mucus assessed by surface plasmon resonance (n=3, mean±SD). The arrow indicates the timing of nanoparticle injection. ***p<0.001 (Student’s t-test). DNA-CP, DNA-loaded conventional particles; DNA-MPP, DNA-loaded mucus-penetrating particles; GFP, green fluorescent protein; shENaC, short hairpin RNA against ENaC.
We next investigated in vivo behaviours of DNA-MPP in comparison to DNA-CP in the lungs of a transgenic mouse model that recapitulates the phenotypic hallmarks of muco-obstructive lung diseases, including mucus plugging and chronic inflammation (ie, Scnn1b-Tg).20 We first compared lung distribution of Cy3-labelled DNA-CP and DNA-MPP following a single intratracheal administration via a microsprayer. One hour after administration, a portion of both formulations was found entrapped within the intraluminal mucus plugs present in the proximal airways of Scnn1b-Tg mice (figure 2A–C). However, while DNA-CP were sparsely distributed or rarely observed along the airway epithelium (figure 2B), DNA-MPP exhibited widespread and uniform distribution throughout the airways and alveolar regions (figure 2C). We next investigated whether the distribution of transgene expression paralleled the observed particle distribution. To this end, Scnn1b-Tg mice were treated with a single dose of DNA-CP or DNA-MPP carrying GFP-encoding plasmids and assessed the transgene expression at 48 hours post-administration via confocal microscopy of lung tissue sections (figure 2D–F). Congruent with the particle distribution profile, GFP transgene expression was sporadically distributed in the lungs of mice treated with DNA-CP (figure 2E), whereas DNA-MPP provided widespread transgene expression throughout the lung epithelium (figure 2F), even in an entire lobe in several cases (online supplemental figure 1). Of note, no fluorescence was observed in untreated lungs (figure 2A,D). Image-based quantification revealed that the airway coverages of GFP transgene expression mediated by DNA-CP and DNA-MPP were ~5% and ~35% (ie, % GFP positive over total epithelial surface), respectively (figure 2G). Likewise, DNA-MPP exhibited approximately eightfold greater coverage of transgene expression compared with DNA-CP in the alveolar region (figure 2H).
Figure 2.
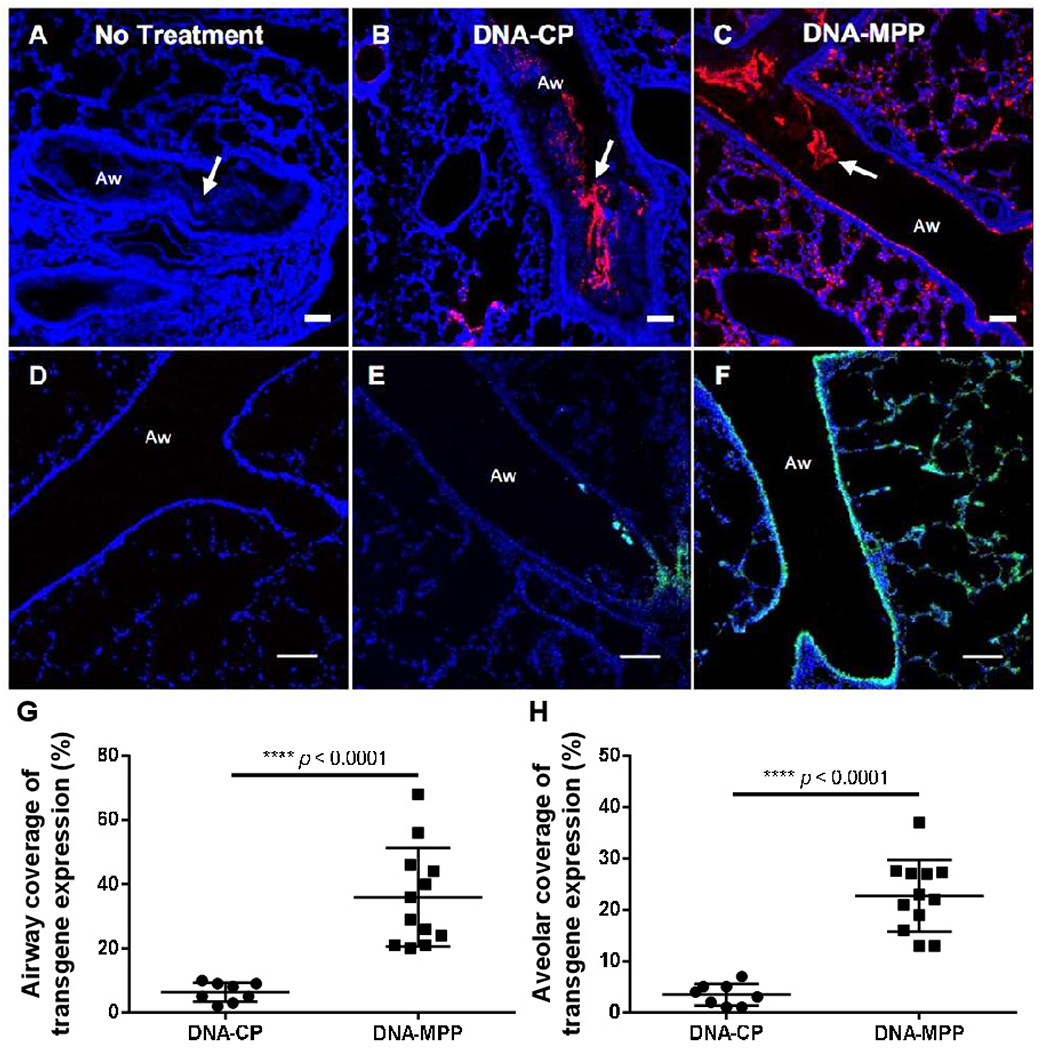
In vivo particle distribution and transgene expression in the lungs of Scnn1b-Tg mice following a single intratracheal administration of DNA-loaded nanoparticles. Representative confocal images demonstrating particle distribution in (A) untreated lung and lungs treated with (B) DNA-CP or (C) DNA-MPP. Red: DNA-CP or DNA-MPP. Blue: cell nuclei (DAPI). White arrows: mucus plugs. Representative confocal images demonstrating reporter transgene expression in (D) untreated lung and lungs treated with (E) DNA-CP or (F) DNA-MPP. Green: GFP transgene expression. Blue: cell nuclei. Aw: airway. Scale bars: 100 μm. Quantification of transgene expression coverage in (G) airways and (H) alveolar regions. Data represent mean±SD (n=8 for DNA-CP, n=12 for DNA-MPP). ****p<0.0001 (Student’s t-test). DAPI, 4′,6-diamidino-2-phenylindole; DNA-CP, DNA-loaded conventional particles; DNA-MPP, DNA-loaded mucus-penetrating particles; GFP, green fluorescent protein.
We subsequently investigated whether the widespread gene transfer enabled by DNA-MPP resulted in high-level overall transgene expression. Specifically, DNA-CP or DNA-MPP carrying luciferase-encoding plasmids (at 0.5 mg/mL plasmids in 50 μL) were administered intratracheally to the lungs of Scnn1b-Tg mice, and the lung tissues were harvested for homogenate-based luciferase assay 7 days after the administration. We found that DNA-MPP delivery resulted in about an order of magnitude greater luciferase activity compared with the level achieved by DNA-CP (figure 3A). We then conducted a dose escalation study where single incrementing doses of DNA-MPP carrying luciferase-encoding plasmids (ie, 0.125 mg/mL to 2 mg/mL plasmids in 50 μL) provided dose-dependent increase in the luciferase activity (figure 3B). While the 1 mg/mL plasmid concentration provided significantly greater overall transgene expression (ie, luciferase activity) compared with its half dose (ie, 0.5 mg/mL) (figure 3B), we elected to conduct further studies with the latter due to the in vivo safety profile we established in healthy mice in our prior study.10 We next evaluated the effect of repeated administration of DNA-MPP and discovered that two doses given at a 1-week interval resulted in statistically significant amplification of the overall transgene expression level (figure 3C). Next, we tested whether DNA-MPP demonstrated an acceptable safety profile in relevant disease conditions, such as in readily inflamed lungs of patients with muco-obstructive lung diseases. Specifically, total cell counts were quantified in BAL fluid harvested from Scnn1b-Tg mice after the DNA-MPP administration. Consistent with a prior observation,20 the total cell count was markedly greater in the lungs of Scnn1b-Tg mice compared with the lungs of littermate wild-type control mice (figure 3D). However, a single or two consecutive dose(s) of DNA-MPP did not exacerbate the chronic inflammation in the lungs of Scnn1b-Tg mice, as evidenced by the virtually identical total cell counts (figure 3D). In addition, H&E staining of lung tissues from different treatment groups further indicated the in vivo safety of DNA-MPP in the inflamed lungs (figure 3E–H).
Figure 3.
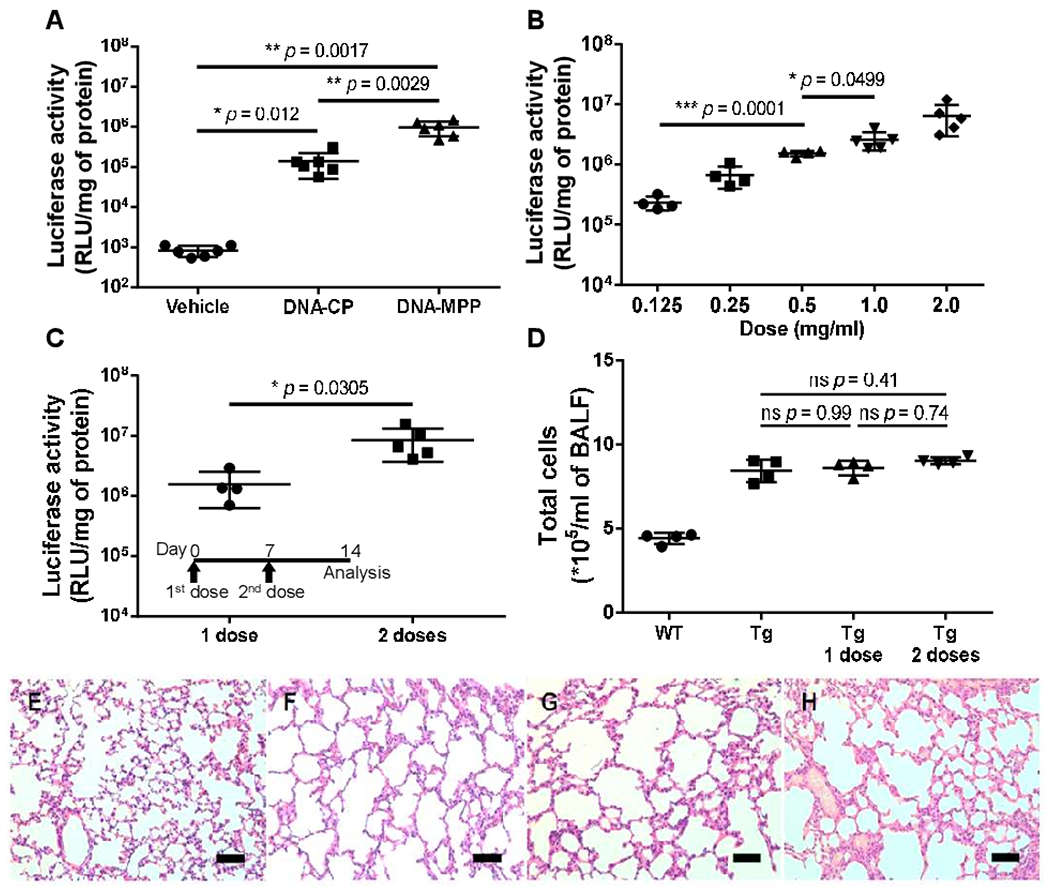
Overall level of in vivo transgene expression in the lungs of Scnn1b-Tg mice following intratracheal administration of DNA-loaded nanoparticles. (A) Comparison of transgene expression mediated by DNA-CP and DNA-MPP following a single administration (n=6, mean±SD). *p<0.05, **p<0.01 (one-way ANOVA). (B) Transgene expression mediated by incrementing doses of DNA-MPP (n=4–5, mean±SD). *p<0.05, ***p<0.001 (one-way ANOVA). (C) Transgene expression mediated by repeated doses of DNA-MPP (n=4–5, mean±SD). *p<0.05 (Student’s t-test). (D) Total number of cells in BAL fluid following a single and repeated treatment(s) with DNA-MPP (n=4, mean±SD). The differences are not statistically significant as indicated (one-way ANOVA). Histological H&E staining of lungs of (E) untreated WT littermate mice, (F) untreated Scnn1b-Tg mice and Scnn1b-Tg mice received (G) a single dose or (H) two doses of DNA-MPP. Scale bar: 200 μm. ANOVA, analysis of variance; BAL, bronchoalveolar lavage; DNA-CP, DNA-loaded conventional particles; DNA-MPP, DNA-loaded mucus-penetrating particles; RLU, relative light unit; Tg, transgenic; WT, wild type.
We next investigated whether DNA-MPP were capable of delivering therapeutic plasmids and ameliorate pathological phenotypes in the lungs of Scnn1b-Tg mice. Specifically, we treated mice with DNA-MPP carrying plasmids encoding shRNA against α subunit of ENaC (shENaC-MPP), after confirming the ability of the plasmid to downregulate ENaC in vitro (online supplemental figure 2). While the diseased phenotypes observed in the lungs of the Scnn1b-Tg mice are driven by overexpression of β subunit of ENaC,20 we opted to target the α subunit due to its essential role on ENaC physiology.23–25 We also note that the α subunit is currently serving as a primary clinical target for ENaC-based CF therapy.26 27 Western blot analysis revealed that a single dose of shENaC-MPP was capable of mediating significant downregulation of both glycosylated and non-glycosylated forms of ENaC (figures 3 and 4A,B). As expected from the earlier reporter multidose study (figure 3C), we also confirmed that two consecutive doses of shENaC-MPP further reduced ENaC expression (online supplemental figure 4). Quantitatively, a single and two dose(s) resulted in 30% and 60% ENaC downregulation, respectively, compared with the vehicle-treated control mice (figure 4C). We hypothesised that the shENaC-MPP-mediated ENaC downregulation would reduce the amount of mucus plugs in the lung airways of Scnn1b-Tg mice via restoring the MCC. We thus went on to collect and assess the amount of mucus plugs in BAL fluid harvested from each mouse, but the quantity was immeasurable by a conventional laboratory analytical balance. Alternatively, we quantified the major component in the mucus plugs, mucin, and found that approximately 45% or 60% reduction of mucin content compared with the vehicle-treated control was observed when shENaC-MPP were administered once or twice, respectively (figure 4D). In parallel, we conducted a control experiment in which we confirmed that the mucin concentration in the lungs of Scnn1b-Tg mice remained unchanged when mice received DNA-MPP carrying GFP-encoding plasmids instead of shENaC-MPP (online supplemental figure 5).
Figure 4.
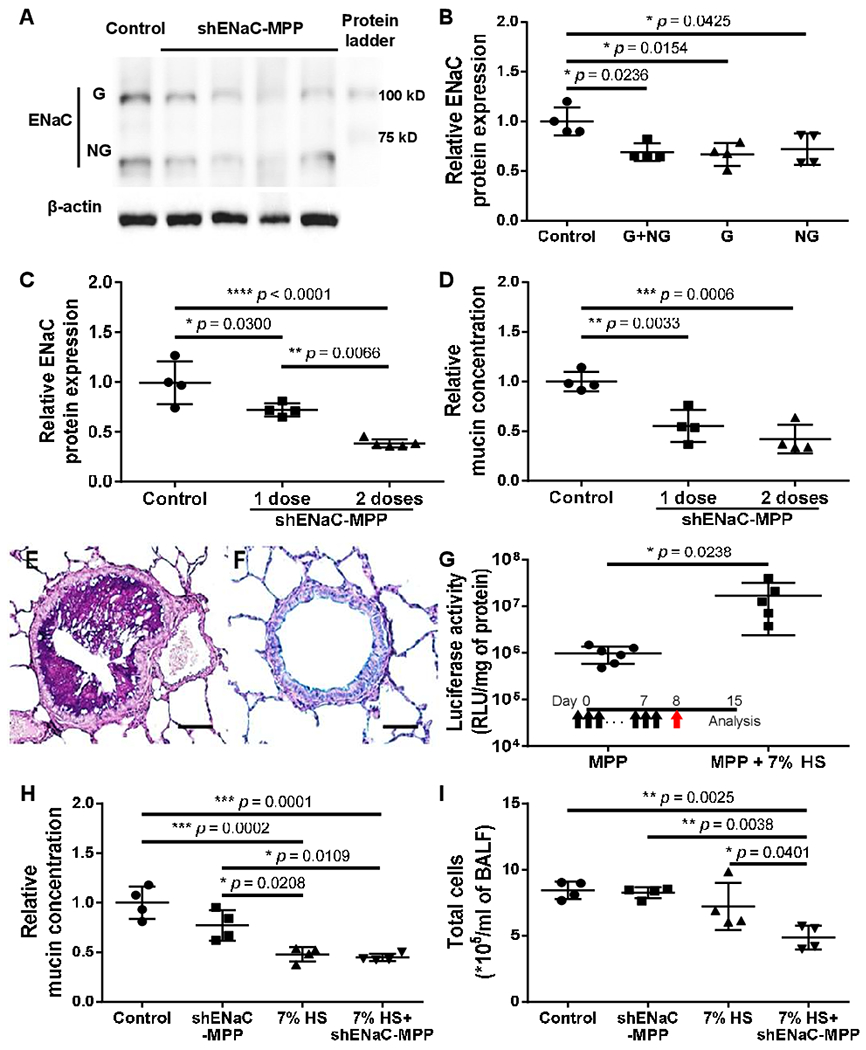
Clinically relevant effectiveness of DNA-MPP carrying shENaC-encoding plasmids (shENaC-MPP) in the lungs of Scnn1b-Tg mice. Western blot (A) imaging and (B) quantitative analysis demonstrating downregulation of ENaC in the lungs of Scnn1b-Tg mice by a single intratracheal dose of shENaC-MPP (n=4, mean±SD). G: glycosylated. NG: non-glycosylated. *p<0.05 (one-way ANOVA). (C) Further downregulation of ENaC after repeated doses of shENaC-MPP (n=4–5, mean±SD). *p<0.05, **p<0.01, ****p<0.0001 (one-way ANOVA). (D) Relative total mucin concentration in BAL fluids collected from untreated Scnn1b-Tg mice vs Scnn1b-Tg mice received a single or two dose(s) of shENaC-MPP (n=4, mean±SD). **p<0.01, ***p<0.001 (one-way ANOVA). Ab-PAS staining of Scnn1b-Tg mouse lungs treated with (E) vehicle (ie, ultrapure water) or (F) 7% HS. Scale bar, 500 μm. (G) Enhancement of DNA-MPP-mediated overall transgene expression in the lungs of Scnn1b-Tg mice received 7% HS pretreatment (1 μL/g body weight, 3 times/day, 7 days) (n=5–6, mean±SD). Inset shows treatment regimen (black arrows: 7% HS pretreatment; red arrow: DNA-MPP administration). *p<0.05 (Student’s t-test). (H) Relative total mucin concentration and (I) total number of cells in BAL fluids collected from Scnn1b-Tg mice received single dose of shENaC-MPP, 7% HS, or sequential treatments of 7% HS and shENaC-MPP in comparison to untreated Scnn1b-Tg mouse control (n=4, mean±SD). *p<0.05, **p<0.01, ***p<0.001 (one-way ANOVA). Ab-PAS, Alcian blue/Periodic acid-Schiff; ANOVA, analysis of variance; BAL, bronchoalveolar lavage; DNA-MPP, DNA-loaded mucus-penetrating particles; ENaC, epithelial sodium channel; G, glycosylated; HS, hypertonic saline; NG, non-glycosylated; RLU, relative light unit; shENaC, short hairpin RNA (shRNA) against ENaC would; Tg, transgenic.
Hypertonic saline (HS; 7% NaCl) is routinely used clinically to improve pulmonary function in patients with CF by facilitating mucus clearance.28 We thus hypothesised that pretreatments with HS would improve gene transfer efficacy of inhaled DNA-MPP by mitigating the mucus burden, improving their access to the airway epithelium. Consistent with a prior observation,29 1-week regimen of intranasal HS (three times a day; 1 μL/g of body weight) was highly efficient in removing mucus plugs in the lungs of Scnn1b-Tg mice (figure 4E,F). We then established that the HS pretreatments further enhanced DNA-MPP-mediated transgene expression (ie, luciferase activity) by over an order of magnitude (figure 4G). Encouraged by this result, we investigated the combined therapeutic effects of HS pretreatments followed by a single dose of shENaC-MPP on key pathology in the lungs of Scnn1b-Tg mice, including muco-obstruction and inflammation. A modest reduction in the mucin concentration was observed in the lungs of Scnn1b-Tg mice received a single dose of shENaC-MPP However, 1-week regimen of intranasal HS was more effective in alleviating the mucus burden, whereas subsequent treatment with shENaC-MPP did not lead to further reduction in the mucin concentration (figure 4H). We note that unlike the earlier study (figure 4D), we quantified the mucin concentration in the whole BAL fluid rather than in mucus plugs, which are cleared in the lungs of HS-treated mice. Interestingly, the total cell count in BAL fluid was significantly reduced in the lungs of mice that received sequential HS pretreatments and shENaC-MPP whereas either treatment alone failed to do so (figure 4I).
To evaluate the relevance of DNA-MPP delivery to human airway epithelial cells in the context of muco-obstructive lung disease, we next established air–liquid interface (ALI) cultures with primary CF human bronchial epithelial (CF HBE) cells harvested from an F508del homozygous patient with CF. After a confluent and tight monolayer and mucus secretion were established,30 31 we treated the ALI cultures apically with either DNA-CP or DNA-MPP carrying GFP-encoding plasmids. Of note, each well received a very small volume (0.1 mg/mL plasmids in 8 μL) to minimise dilution of the apical mucus gel layer.30 Confocal microscopy revealed that while the transgene expression mediated by DNA-CP was negligible (figure 5A), relatively widespread GFP signal was observed in epithelial monolayers that received DNA-MPP (figure 5B). Quantitatively, DNA-MPP provided a greater level (ie, GFP intensity; figure 5C) and coverage (figure 5D) of transgene expression compared with DNA-CP.
Figure 5.
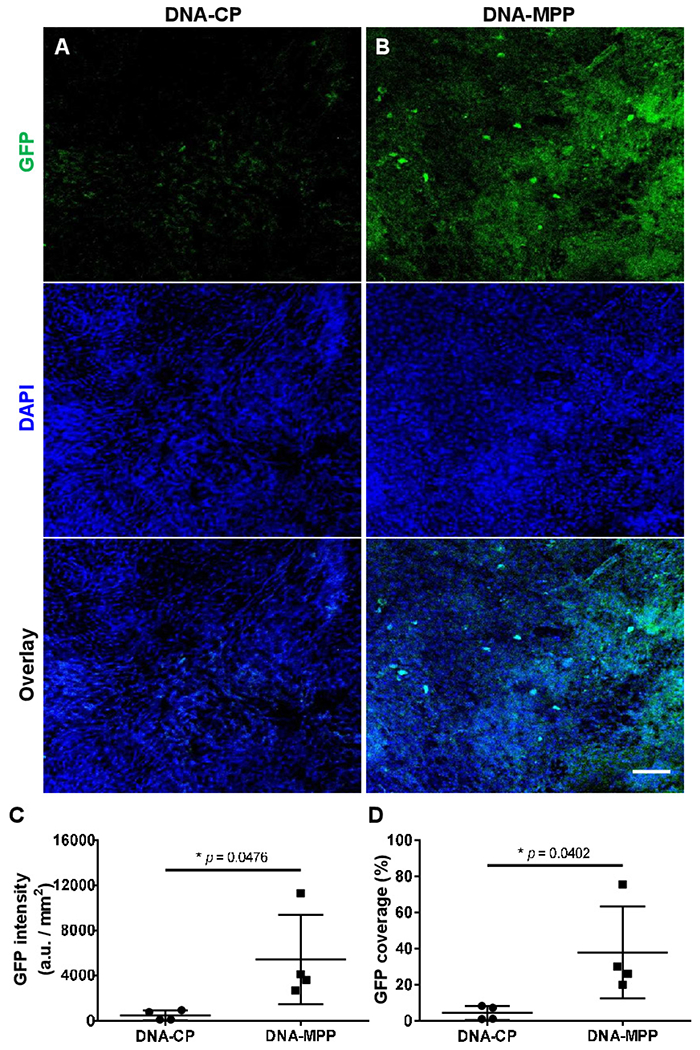
In vitro transgene expression in ALI cultures of primary CF HBE cells. Representative confocal images of GFP transgene expression mediated by (A) DNA-CP or (B) DNA-MPP. Scale bar: 100 μm. Image-based quantification of (C) level and (D) coverage of GFP transgene expression mediated by DNA-CP vs DNA-MPP (n=4, mean±SD). *p<0.05 (Student’s t-test). ALI, air-liquid interface; CF, cystic fibrosis; DAPI, 4′,6-diamidino-2-phenylindole; DNA-CP, DNA-loaded conventional particles; DNA-MPP, DNA-loaded mucus-penetrating particle; GFP, green fluorescent protein; HBE, human bronchial epithelial.
MATERIALS AND METHODS
Please refer to the online supplemental material 1
DISCUSSION
We here demonstrate that a synthetic gene delivery platform uniquely designed to efficiently penetrate the airway mucus gel layer (ie, DNA-MPP) provides widespread reporter transgene expression throughout the lungs of a mouse model of muco-obstructive lung diseases (ie, Scnn1b-Tg). Our image-based analysis revealed that DNA-MPP provided at least 30% airway coverage of transgene expression on average in the lungs of Scnn1b-Tg mice and ALI cultures of primary human CF bronchial epithelium. In contrast, an otherwise identical mucus-impermeable formulation (ie, DNA-CP) exhibited less than 10% coverage in these highly relevant CF airway models. Encouragingly, it has been demonstrated using an in vitro model of human primary CF ciliated airway epithelium that functional CF transmembrane conductance regulator protein production in 25% of cells restore normal mucus transport rates.32 We acknowledge that a next step in the line of this study would be confirmation of the findings using a more quantitative method, such as flow cytometry, and ultimately demonstration of bioactivity of the transgene.
Although the widespread coverage mediated by DNA-MPP resulted in a high-level transgene expression in the lungs of Scnn1b-Tg mice, the levels of expression were still an order of magnitude lower compared with an adeno-associated virus (AAV) serotype that we previously confirmed for efficient mucus penetration (ie, AAV6).30 However, we found in this study that two doses of DNA-MPP markedly enhanced the level of transgene expression compared with a single administration, underscoring that our formulation did not elicit therapy-inactivating immunogenicity inevitably observed with virus-based gene vectors.33 To this end, DNA-MPP may provide transgene expression levels on par or greater than mucus-penetrating viral vectors by virtue of multiple dosing regimens. We have previously shown the DNA-MPP does not trigger acute inflammatory responses in the lungs of healthy mice,10 but it is critical to retain this safety profile in diseased lungs. We thus demonstrate here that DNA-MPP does not exacerbate the chronic inflammation already present in the muco-obstructed lungs of Scnn1b-Tg mice.20 34 This finding suggests that DNA-MPP would be safe even in the immunologically perturbed environment inherent to muco-obstructive lung diseases.
We discovered that a single dose of shENaC-MPP markedly reduced the level of ENaC protein production in the lungs of Scnn1b-Tg mice. This effect was dose dependent and further pronounced on repeated administration. Importantly, the mucus burden was significantly reduced by the shENaC-MPP-mediated downregulation of ENaC, presumably due to airway rehydration followed by partial restoration of MCC. In support of the scenario, it has been recently reported that three times daily intranasal administration of ENaC-inhibitory peptides significantly increases the airway surface liquid (ASL) height (ie, airway rehydration) in the airways of neonatal Scnn1b-Tg mice.35 Likewise, ENaC antagonists have been shown to restore and/or increase the ASL height in ALI cultures of human CF bronchial epithelial cells.35 36 Of note, Zhou et al37 demonstrated that a small molecule-based ENaC inhibitor, amiloride, given at an identical dosing schedule applied to the aforementioned peptides prevented mucus obstruction in the airways of neonatal Scnn1b-Tg mice, but therapeutic reversion was not achieved with adult mice. Remarkably, we demonstrate here a moderate but significant mitigation of mucus plugging in the lungs of Scnn1b-Tg mice treated with shENaC-MPP at an age of 4 weeks when muco-obstructive phenotype is fully established.20 34 This demonstrates the potential advantage of genetic knockdown of ENaC, as opposed to small molecules or other therapies that may not completely engage the target.
We report that pre-treatment with HS markedly increases the overall level of transgene expression mediated by subsequent administration of DNA-MPP. Of note, an appreciable amount of DNA-MPP despite the excellent mucus-penetrating ability,10 was found within the mucus plugs in the airways of Scnn1b-Tg mice that did not receive the pretreatment. Thus, the further enhancement of transgene expression is most likely attributed to the significant reduction of the particle-trapping mucus plugs via the pretreatment as evidenced by our histological analysis and a prior report.29 We found that the airway-rehydrating HS alone failed to ameliorate chronic inflammation inherent to the model, in agreement with prior observations with both Scnn1b-Tg mice29 and patients with CF.28 Furthermore, even a remarkable ENaC downregulation achieved by shENaC-MPP was insufficient to alter the inflammatory profile, likely due to the proinflammatory effect of resident mucus plugs, as recently demonstrated in ferrets with sterile mucus obstruction.38 However, a combinatory regimen of HS and shENaC-MPP roughly halved the inflammatory burden in the lungs of Scnn1b-Tg mice. The findings here suggest that a threshold of ENaC downregulation required for airway rehydration relevant to intervening the chronic inflammation may exist at least in this model. Of note, a synergistic effect of HS and ENaC inhibition on airway rehydration, but not chronic inflammation, has been previously reported using a preclinical model.39
It has been previously reported that relative airway epithelial gene transfer efficacies of different gene vectors vary with species.40 Thus, despite the relevance and value of advanced animal models, clinical development of lung gene therapy mandates validation of gene delivery strategies using human primary airway cells from patients with muco-obstructive lung diseases. Specifically, ALI cultures recapitulate several key delivery barriers found in lung airways in vivo,1 but have been extremely difficult to transfect particularly when it comes to non-viral gene delivery systems. Encouragingly, a few lipid-based gene vectors have recently demonstrated abilities to mediate meaningful therapeutic transgene expression or gene silencing in ALI cultures established with CF HBE cells.41 42 However, these studies used patient-derived immortalised cell lines (eg, 16HBE14o-) that generally do not differentiate fully, or impact of the physiological mucus barrier was not factored in. While virus-based gene vectors have been more widely tested in ALI cultures of primary CF HBE cells, a majority of studies involve the use of large volumes that essentially dilute and alter the apically established mucus gel layer.43–47 Here, we report that a small volume of apically administered DNA-MPP is capable of mediating widespread transgene expression in mucus-covered ALI cultures of CF HBE cells directly harvested from a patient with CF, in agreement with our in vivo observation in the lungs of Scnn1b-Tg mice.
Supplementary Material
Key messages.
What is the key question?
Can we demonstrate an effective inhaled gene therapy modality in preclinical models that closely recapitulate the hallmarks of muco-obstructive lung diseases?
What is the bottom line?
DNA-loaded mucus-penetrating particle is capable of efficiently penetrating pathological airway mucus barrier, thereby providing highly efficient gene transfer efficacy and therapeutic potentials in highly relevant models of muco-obstructive lung diseases.
Why read on?
Clinical implementation of inhaled gene therapy of muco-obstructive lung diseases is yet to be achieved largely due to inability to breach the pathological mucus gel layer covering the lung airways. We here demonstrate that efficient mucus penetration is critical to achieving widespread and robust gene transfer to mucus-laden airway epithelium and providing therapeutic benefits in a preclinical model of muco-obstructive lung diseases.
Funding
The funding was provided by the National Institute of Health (NIH) (R01HL127413, R01HL136617, P30DK072482 and P30EY001765) and the Cystic Fibrosis Foundation (SUK18I0 and ROWE19R0). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests
The mucus-penetrating particle technology described in this publication has been developed at the Johns Hopkins University and is currently licensed to Kala Pharmaceuticals. JH is a founder of Kala Pharmaceuticals and currently serves as a consultant. JH and Johns Hopkins University own company stock; JH’s relationship with Kala Pharmaceuticals is subject to certain restrictions under university policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
Patient consent for publication Not required.
Ethics approval The animal experiments in this study were monitored and approved by Johns Hopkins University’s Institutional Animal Care and Use Committee.
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/thoraxjnl-2020-215185).
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- 1.Kim N, Duncan GA, Hanes J, et al. Barriers to inhaled gene therapy of obstructive lung diseases: a review. J Control Release 2016;240:465–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Z, McCray PB, Engelhardt JF. Advances in gene therapy for cystic fibrosis lung disease. Hum Mol Genet 2019;28:R88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Liu J, Wu J, et al. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin Drug Deliv 2021;18:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisholm JF, Shenoy SK, Shade JK, et al. Nanoparticle diffusion in spontaneously expectorated sputum as a biophysical tool to probe disease severity in COPD. Eur Respir J 2019;54. doi: 10.1183/13993003.00088-2019. [Epub ahead of print: 01 08 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev 2009;61:75–85. [DOI] [PubMed] [Google Scholar]
- 6.Duncan GA, Jung J, Hanes J, et al. The mucus barrier to inhaled gene therapy. Mol Ther 2016;24:2043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesimer M, Ford AA, Ceppe A, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017;377:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hida K, Lai SK, Suk JS, et al. Common gene therapy viral vectors do not efficiently penetrate sputum from cystic fibrosis patients. PLoS One 2011;6:e19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster BS, Kim AJ, Kays JC, et al. Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol Ther 2014;22:1484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A 2015;112:8720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders NN, De Smedt SC, Demeester J. Mobility and stability of gene complexes in biogels. J Control Release 2003;87:117–29. [DOI] [PubMed] [Google Scholar]
- 12.Suk JS, Kim AJ, Trehan K, et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release 2014;178:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suk JS, Lai SK, Wang Y-Y, et al. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009;30:2591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Chisholm J, Zhuang J, et al. Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci U S A 2017;114:E6595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman G, Rodriguez J, Chan SY, et al. Pegylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J Control Release 2018;285:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider CS, Xu Q, Boylan NJ, et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv 2017;3:e1601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva AL, de Oliveira GP, Kim N, et al. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci Adv 2020;6:eaay7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Liu S, Chen D, et al. A two-pronged pulmonary gene delivery strategy: a surface-modified fullerene nanoparticle and a hypotonic vehicle. Angew Chem Int Ed Engl 2021;60:15225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YC, Hsueh HT, Kim N. Strategy to enhance dendritic cell-mediated DNA vaccination in the lung. Adv Ther-Germany 2020;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mall M, Grubb BR, Harkema JR, et al. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004;10:487–93. [DOI] [PubMed] [Google Scholar]
- 21.Mall MA, Galietta LJV. Targeting ion channels in cystic fibrosis. J Cyst Fibros 2015;14:561–70. [DOI] [PubMed] [Google Scholar]
- 22.McCarron A, Donnelley M, Parsons D. Airway disease phenotypes in animal models of cystic fibrosis. Respir Res 2018;19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol 1994;267:C1682–90. [DOI] [PubMed] [Google Scholar]
- 24.Hummler E, Barker P, Gatzy J, et al. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 1996;12:325–8. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Crosby J, Lv T, et al. Antisense oligonucleotide targeting of mRNAs encoding ENaC subunits α, β, and γ improves cystic fibrosis-like disease in mice. J Cyst Fibros 2019;18:334–41. [DOI] [PubMed] [Google Scholar]
- 26.Mall MA, Danahay H, Boucher RC. Emerging concepts and therapies for Mucoobstructive lung disease. Ann Am Thorac Soc 2018;15:S216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore PJ, Tarran R. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease. Expert Opin Ther Targets 2018;22:687–701. [DOI] [PubMed] [Google Scholar]
- 28.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006;354:229–40. [DOI] [PubMed] [Google Scholar]
- 29.Graeber SY, Zhou-Suckow Z, Schatterny J, et al. Hypertonic saline is effective in the prevention and treatment of mucus obstruction, but not airway inflammation, in mice with chronic obstructive lung disease. Am J Respir Cell Mol Biol 2013;49:410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan GA, Kim N, Colon-Cortes Y, et al. An adeno-associated viral vector capable of penetrating the mucus barrier to inhaled gene therapy. Mol Ther Methods Clin Dev 2018;9:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birket SE, Rowe SM. Revealing the molecular signaling pathways of mucus stasis in cystic fibrosis. J Clin Invest 2019;129:4089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Button B, Gabriel SE, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 2009;7:e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill DR, Hyde SC. Delivery of genes into the CF airway. Thorax 2014;69:962–4. [DOI] [PubMed] [Google Scholar]
- 34.Mall MA, Harkema JR, Trojanek JB, et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 2008;177:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terryah ST, Fellner RC, Ahmad S, et al. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol 2018;314:L192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sesma JI, Wu B, Stuhlmiller TJ, et al. SPX-101 is stable in and retains function after exposure to cystic fibrosis sputum. J Cyst Fibros 2019;18:244–50. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Z, Treis D, Schubert SC, et al. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am J Respir Crit Care Med 2008;178:1245–56. [DOI] [PubMed] [Google Scholar]
- 38.Rosen BH, Evans TIA, Moll SR, et al. Infection is not required for Mucoinflammatory lung disease in CFTR-Knockout ferrets. Am J Respir Crit Care Med 2018;197:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blé F-X, Cannet C, Collingwood S, et al. ENaC-mediated effects assessed by MRI in a rat model of hypertonic saline-induced lung hydration. Br J Pharmacol 2010;160:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Luo M, Trygg C, et al. Biological differences in rAAV transduction of airway epithelia in humans and in old world non-human primates. Mol Ther 2007;15:2114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson E, MacDonald KD, Slaughter K, et al. Lipid Nanoparticle-Delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther 2018;26:2034–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tagalakis AD, Munye MM, Ivanova R, et al. Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax 2018;73:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bals R, Xiao W, Sang N, et al. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol 1999;73:6085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limberis MP, Vandenberghe LH, Zhang L, et al. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther 2009;17:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitomo K, Griesenbach U, Inoue M, et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther 2010;18:1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Z, Keiser NW, Song Y, et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther 2013;21:2181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Z, Zak R, Zhang Y, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 2004;78:2863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


