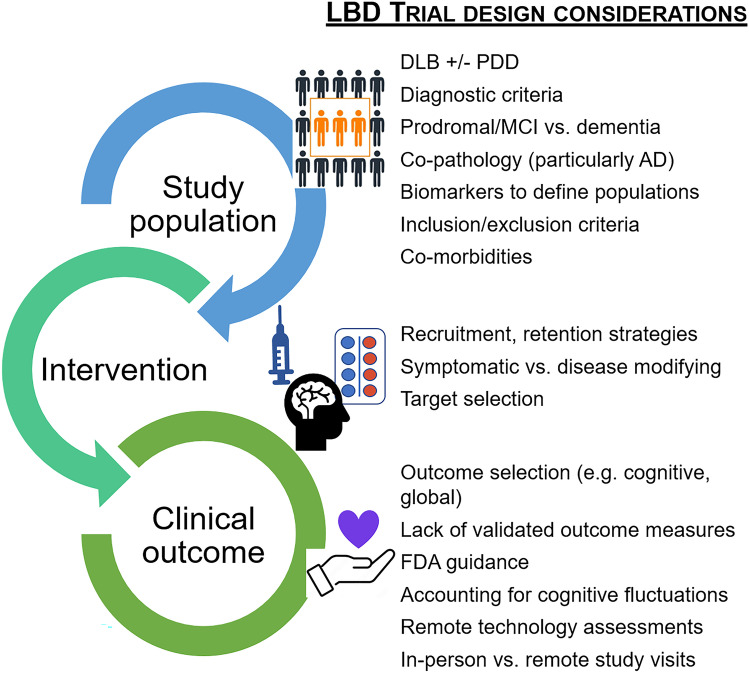
Fig. 2.
Challenges facing Lewy body dementia (LBD) trial design. Clinical trials and drug development are top research priorities in LBD, but many special considerations make trial development and implementation particularly challenging for the LBD population. Researchers and trial designers need to thoughtfully select the appropriate study population, intervention, and outcome measures. There are many opportunities to improve LBD trial design. Research efforts are underway to look for better biomarkers (e.g., aid diagnosis, verify target engagement, monitor disease progression and therapeutic responses), to develop and validate LBD-specific outcome measures, and to innovate alternative assessment strategies (e.g., telemedicine, home-based technologies, serial testing) that can account for cognitive fluctuations and reduce study visit burden. Successful LBD trials will require collaborative and multifaceted approaches