FIGURE 5.
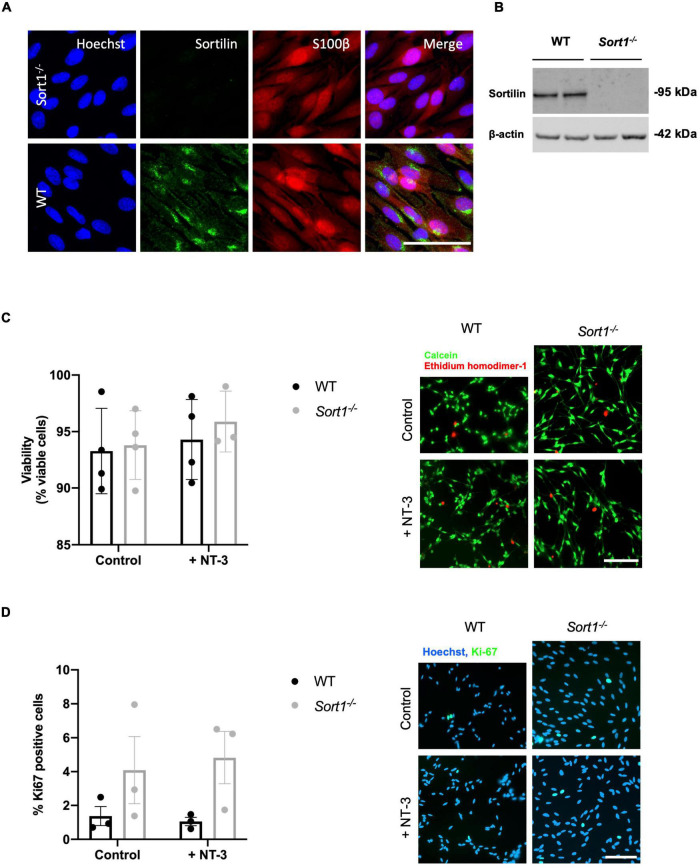
Primary cultured Schwann cells express sortilin. (A) Double-labeled immunofluorescence microscopy of primary cultured Schwann cells show sortilin (green) in wild-type (WT) Schwann cells (red; identified by the Schwann cell marker s100β), but not in cells derived from Sort1–/– rat sciatic nerves. Nuclei are labeled with Hoechst (blue). Scale bar 50 μm. (B) Immunoblot analysis of sortilin in lysates of WT or Sort1–/– primary Schwann cells shows sortilin expression in WT. (C) The viability of Sort1–/– Schwann cells is like WT Schwann cells with (95.89 ± 1.55% vs. 94.30 ± 1.77%, p > 0.99) or without NT-3 stimulation (93.8 ± 1.52% vs. 93.29 ± 1.89%, p > 0.99). Viable cells were identified in Schwann cell cultures 24 h after stimulation with or without NT-3 by calcein (green) fluorescence, while dead cells were identified by ethidium homodimer-1 (red) fluorescence. Scale bar 100 μm. Viability reflects mean% calcein-positive cells ± SEM, n = 4. (D) Schwann cell proliferation (percentage of Ki-67% cells) was not significantly different in cultures of Sort1–/– Schwann cells compared to WT Schwann cell cultures with (4.82 ± 1.54% vs. 1.05 ± 0.25%, p = 0.4381) or without NT-3 stimulation (4.09 ± 1.98% vs. 1.37 ± 0.56%, p > 0.99). The proliferation marker, Ki-67 (green), was identified by immunofluorescence in Schwann cell cultures 48 h after stimulation with or without NT-3. Nuclei are labeled with Hoechst (blue). Scale bar 100 μm. Proliferation rates reflect% Ki-67 positive cells ± S.E.M., n = 3. Statistical significance was analyzed using two-way ANOVA with Bonferroni’s multiple comparisons post hoc test.