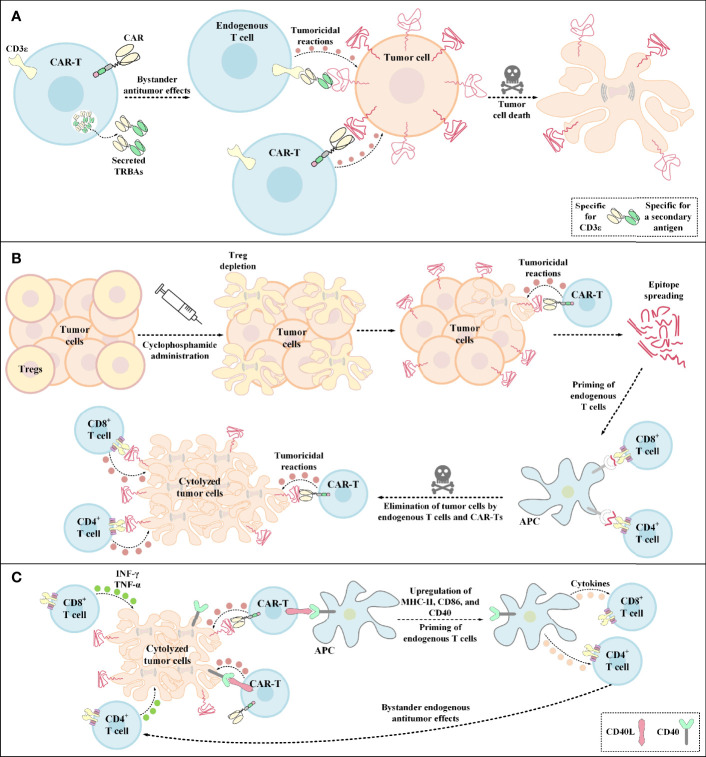
Figure 4.
Bystander antitumor effect induction by TRBA-secreting CAR-Ts, cyclophosphamide administration, and CD40L-expressing CAR-Ts. (A) TRBA-secreting CAR-Ts. TRBAs are made of two scFvs fused via a linker peptide. One of these scFvs targets CD3 (present on the surface of endogenous T cells) and the other one targets a TAA or TSA of interest against which endogenous T-cell responses are intended to be redirected. TRBA-secreting CAR-Ts secrete these bispecific T-cell-redirecting antibodies which results in endogenous T-cell-mediated antitumor reactions against malignant cells alongside CAR-T-mediated tumoricidal responses enabling a more effective tumor cell elimination. (B) Cyclophosphamide administration. Cyclophosphamide administration mediates Treg depletion and enables a more efficient CAR-T engagement with its target antigen and the subsequent CAR-T-mediated tumoricidal reactions. Additionally, upon epitope spreading, APCs uptake the released peptide antigens and present them to CD4+ T cells and CD8+ T cells. This mechanism leads to the priming of endogenous T cells and the subsequent elimination of tumor cells through bystander antitumor effects mediated by these endogenous cells. (C) The mechanism of action of CD40L+ CAR-Ts. CD40L+ CAR-Ts can mediate tumor cell cytolysis through both their CAR and their CD40L interacting with the CAR target antigen and CD40 on the surface of tumor cells, respectively. Additionally, CD40L+ CAR-Ts mediate DC licensing as indicated by the upregulated level of CD40, CD86, and MHC-II. These APCs in turn recruit other immune effector cells such as endogenous T cells. INF-γ and TNF-α secretion by the recruited endogenous CD4+ T cells and CD8+ T cells also result in tumor cell cytolysis. APC, antigen-presenting cells; CAR, chimeric antigen receptor; CD40L, CD40 ligand; INF-γ, interferon γ; MHC-II, major histocompatibility complex class II; TNF-α, tumor necrosis factor α; TRBAs, T-cell-redirecting bispecific antibodies; Treg, regulatory T cell.