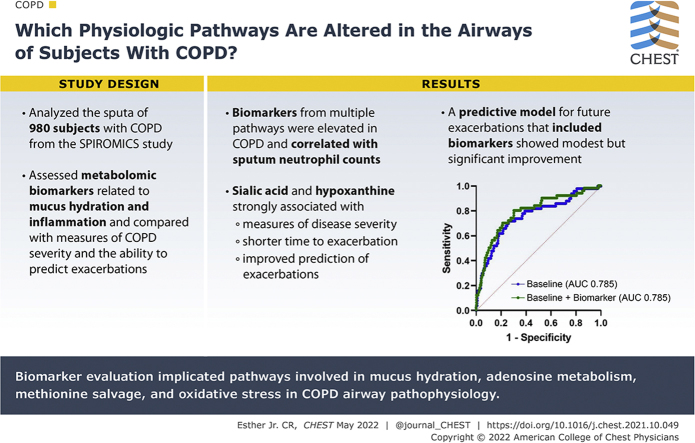
Abstract
Background
Improved understanding of the pathways associated with airway pathophysiologic features in COPD will identify new predictive biomarkers and novel therapeutic targets.
Research Question
Which physiologic pathways are altered in the airways of patients with COPD and will predict exacerbations?
Study Design and Methods
We applied a mass spectrometric panel of metabolomic biomarkers related to mucus hydration and inflammation to sputa from the multicenter Subpopulations and Intermediate Outcome Measures in COPD Study. Biomarkers elevated in sputa from patients with COPD were evaluated for relationships to measures of COPD disease severity and their ability to predict future exacerbations.
Results
Sputum supernatants from 980 patients were analyzed: 77 healthy nonsmokers, 341 smokers with preserved spirometry, and 562 patients with COPD (178 with Global Initiative on Chronic Obstructive Lung Disease [GOLD] stage 1 disease, 303 with GOLD stage 2 disease, and 81 with GOLD stage 3 disease) were analyzed. Biomarkers from multiple pathways were elevated in COPD and correlated with sputum neutrophil counts. Among the most significant analytes (false discovery rate, 0.1) were sialic acid, hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione. Sialic acid and hypoxanthine were associated strongly with measures of disease severity, and elevation of these biomarkers was associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Interpretation
Biomarker evaluation implicated pathways involved in mucus hydration, adenosine metabolism, methionine salvage, and oxidative stress in COPD airway pathophysiologic characteristics. Therapies that target these pathways may be of benefit in COPD, and a simple model adding sputum-soluble phase biomarkers improves prediction of pulmonary exacerbations.
Trial Registry
ClinicalTrials.gov; No.: NCT01969344; URL: www.clinicaltrials.gov
Key Words: adenosine, glutathione, inflammation, metabolomics, methionine salvage, mucus
Abbreviations: AUC, area under the receiver operating characteristic curve; CF, cystic fibrosis; GOLD, Global Initiative on Chronic Obstructive Lung Disease; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study
Graphical Abstract
Take-home Points.
Study Question: Which physiologic pathways are altered in the airways of patients with COPD and predict exacerbations?
Results: Application of a mass spectrometric biomarker panel to supernatants of sputa from patients with COPD and appropriate control participants revealed that sialic acid, hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione were altered in COPD, correlated with measures of disease severity and predictive of pulmonary exacerbations.
Interpretation: Physiologic pathways involved in mucus hydration, adenosine metabolism, methionine salvage, and oxidative stress are implicated in COPD airway pathophysiologic characteristics, with these pathways representing both biomarkers and therapeutic targets.
COPD is characterized by dominant small airways obstruction with associated airway inflammation. Biomarkers of multiple inflammatory pathways are elevated in sputum from patients with COPD,1,2 as are indexes of oxidative stress, including oxidized glutathione and 8-isoprostane.3,4 Although these findings reflect the central role of airway inflammation in COPD, inflammation represents a challenging therapeutic target. Identification of other biological pathways involved in COPD pathogenesis could provide novel biomarkers and therapeutic targets in this disease.
We previously used targeted biomarker and metabolomic approaches to identify small molecule metabolites that correlate with airway inflammation in lung diseases such as cystic fibrosis (CF).5,6 These findings supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory biomarkers coupled to biomarkers of mucus hydration.7 To assess the relevance of these biomarkers in patients with COPD, we applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), an observational longitudinal cohort study that has phenotyped extensively a large number of patients with COPD as well as relevant smoking and nonsmoking control participants.8
Study Design and Methods
Patients
Patients represented a subset of participants in the previously described SPIROMICS cohort8 that included groups of healthy nonsmoking patients and patients with at least a 20-pack-year smoking history. Smokers included those with preserved spirometry as well as those with COPD grouped using Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (GOLD stages 1-3).9 Target sample size of 980 patients was chosen to include all patients whose sputum mucin concentrations were measured via physical methods as reported in a previous study.9 However, because banked sputum supernatants were available for only 748 of those patients, the remaining 232 patients were chosen randomly from the remaining cohort (e-Fig 1). Chronic bronchitis was defined as described previously9 as productive cough on most days for 3 consecutive months for 2 years (classic definition) or as cough and phlegm almost every day several times per week per the St. George’s Respiratory Questionnaire.10 Exacerbations were defined as described previously9 as the number of events of worsening respiratory health requiring therapy with corticosteroids, antibiotics, or both that occurred within 365 days of the initial study visit. The SPIROMICS protocol was approved by the participating universities, and participants provided written informed consent. More information about the study and how to access SPIROMICS data are available at www.spiromics.org.
Sputum Collection and Processing
All samples were collected at the baseline visit. Sputum was induced by inhalation of hypertonic saline and was processed using standardized SPIROMICS protocols as described previously.1 Briefly, sputum samples were weighed and diluted 1:4 with 10% Sputolysin (Calbiochem), rocked at room temperature for 15 min, then diluted 1:4 with 1 mM ethylenediaminetetraacetic acid and rocked for an additional 5 min. Samples were filtered through a 50-μm mesh and were centrifuged at 500 g for 10 min, and the supernatant was aliquoted and frozen at –80 °C until analysis.
Mass Spectrometry
Mass spectrometry was performed as described previously.7 Briefly, isotopically labeled internal standards and formic acid (final concentration, 1%) were added to 100 μL of sputum supernatant. The mixture was incubated at 80 °C for 1 h to cleave terminal sialic acid residues from mucins and was filtered through 10-kD selection filters (EMD Millipore) to remove macromolecules, and 5 μL of processed sample was injected into an ultra-performance liquid chromatography system with an Acquity UPLC HSS T3 C18 column (Waters) using 0.1% formic acid and methanol gradients coupled to a TSQ-Quantum Ultra triple quad mass analyzer (ThermoFinnigan), as described previously.7 Mass spectrometric signals (peak area) were determined via Xcalibur software (Thermo Scientific), with biomarker concentrations determined by standard solutions run in parallel. Background suppression for some analytes was observed in a subset of samples, and biomarkers whose internal standard signal was less than 10% the average of the sample run were excluded from analysis.
Statistical Analyses
Biomarker concentrations were not normally distributed and were log base 10-transformed before analysis, as were sputum neutrophil counts. Differences among patient groups were evaluated by Student t test or the analysis of variance with analysis after test by Tukey multiple comparisons test, with correlations performed using Pearson correlations or linear regression. In the initial screening for relevant biomarkers, the Benjamini-Hochberg procedure was used to control for multiple testing with a false discovery rate of 0.10. For exacerbation predictions, a sputum biomarker score from 0 to 2 was defined based on whether sialic acid, hypoxanthine, or both were elevated, that is, in the top quartile of all samples (> 25 mM for sialic acid, > 1 μM for hypoxanthine). Kaplan-Meier curves were used to assess time to exacerbation in patients grouped by biomarker score, with a log-rank Mantel-Cox test to compare groups and Tukey-Kramer adjustments for post hoc comparisons. Multivariate logistic regression to assess models that predict pulmonary exacerbations was performed as described in the text and was reported as area under the receiver operating characteristic curve (AUC) with 95% CIs, with the likelihood ratio test used to compare logistic regression models. Cross-validated AUCs were calculated using k-fold cross-validation, with k = 10, iterated 10 times. Mean and 95% CIs for cross-validated AUCs were calculated on the test datasets for both models and then compared with the Wilcoxon signed-rank test.
Results
We analyzed cell-free sputum supernatants from 980 patients (Table 1), including samples from 77 healthy nonsmokers, 341 ever smokers with preserved spirometry, and 562 patients with COPD (178 with GOLD stage 1 disease, 303 with GOLD stage 2 disease, and 81 with GOLD stage 3 disease). From the biomarker panel, we found that six metabolites were altered significantly in patients with COPD relative to healthy nonsmokers at a false discovery rate of 0.10: the mucin marker sialic acid, the adenosine metabolites hypoxanthine and xanthine, the methionine salvage pathway metabolites methylthioadenosine and adenine, and the oxidative stress marker glutathione (Table 2). These metabolites also were correlated significantly with sputum neutrophil counts, with the exception of methylthioadenosine and glutathione. For reference, similar analyses are shown for two established biomarkers measured in these samples: sputum neutrophil counts (polymorphonuclear cells per milligram) obtained through standard SPIROMICS protocols and sputum mucin concentrations available for a subset of the analyzed samples from a previously published study.9 The pathways associated with these metabolites and their relationships to markers of COPD disease severity are analyzed in more detail herein.
Table 1.
Patient Demographics
| Variable | Nonsmokers | SPS Group | GOLD Stage Group |
SPIROMICS Cohorta | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| No. of patients | 77 | 341 | 178 | 303 | 81 | 2,973 |
| Age, y | 55.4 ± 10.2 | 59.6 ± 9.5 | 66.4 ± 8.4 | 64.9 ± 7.8 | 64.8 ± 8.3 | 63.0 ± 9.2 |
| Male sex | 45 | 56 | 68 | 64 | 57 | 53 |
| White race | 66 | 62 | 81 | 85 | 77 | 78 |
| FEV1 % predicted | 102.9 ± 12.1 | 98.4 ± 14.0 | 92.6 ± 10.3 | 67.0 ± 8.7 | 44.0 ± 4.3 | 75.0 ± 26.7 |
| FEV1 to FVC % ratio predicted | 103.5 ± 5.2 | 100 ± 6.1 | 83.9 ± 7.0 | 73.5 ± 10.8 | 55.5 ± 11.7 | 80.0 ± 21.4 |
| Current smoker | 0 | 56 | 35 | 40 | 38 | 37 |
| Chronic bronchitis | 5 | 42 | 36 | 54 | 53 | 42 |
Data are presented as percentage or mean ± SD, unless otherwise indicated. GOLD = Global Initiative on Chronic Obstructive Lung Disease; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study; SPS = smokers with preserved spirometry.
Baseline characteristics of full SPIROMICS cohort at the time of the study.
Table 2.
Sputum Biomarker Metabolites by Study Group and Inflammation
| Pathway | Metabolite | No. | Fold Change |
Correlation |
|
|---|---|---|---|---|---|
| SPS vs Nonsmoker Groups | COPD vs Nonsmoker Groups | PMN Cell Count /mg | |||
| Mucus hydration | Sialic acid | 875 | 2.05a | 2.65a | 0.35a |
| Urea | 877 | 1.05 | 1.22 | 0.13 | |
| Purinergic signaling | AMP | 970 | 0.98 | 1.13 | 0.22a |
| Adenosine | 968 | 1.46 | 1.23 | –0.07 | |
| Inosine | 912 | 1.46 | 1.44 | –0.06 | |
| Hypoxanthine | 909 | 1.17 | 1.74a | 0.33a | |
| Xanthine | 916 | 1.37 | 1.81a | 0.33a | |
| Uric acid | 975 | 1.30 | 1.50 | 0.10 | |
| Methionine salvage | Methylthioadenosine | 922 | 1.45 | 1.86a | 0.13 |
| Adenine | 967 | 1.90 | 2.29a | 0.33a | |
| Spermine | 878 | 1.20 | 1.49 | 0.31a | |
| Oxidative Stress | Total glutathione | 965 | 1.19 | 1.62a | 0.12 |
| Protein synthesis/degradation | Phenylalanine | 975 | 1.06 | 1.13 | 0.22a |
| Tyrosine | 971 | 1.09 | 1.17 | 0.11 | |
| Leucine | 974 | 0.99 | 1.20 | 0.34a | |
| Proline | 952 | 1.25 | 1.55 | 0.09 | |
| Leu-pro dipeptide | 899 | 1.18 | 1.52 | 0.30a | |
| Other | Nicotinamide | 723 | 0.99 | 1.19 | 0.21a |
| Taurine | 643 | 1.05 | 1.03 | –0.05 | |
| Established | PMN count | 594 | 1.96 | 3.47a | N/A |
| Mucins | 748 | 1.39 | 1.67a | 0.21a | |
Boldface values indicate significance at P < .05. AMP = adenosine metabolic pathway; PMN = polymorphonuclear; SPS = smokers with preserved spirometry.
Significant after correction for multiple testing (false discovery rate, < 0.01).
Biomarker Analyses by Disease Severity
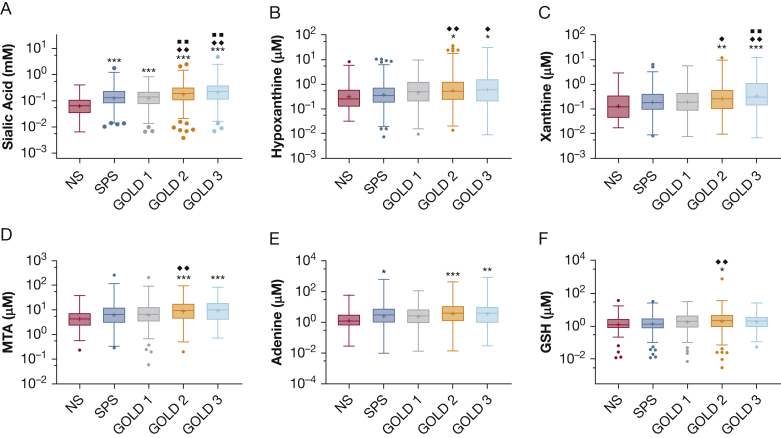
To assess relationships to the GOLD measure of disease severity, each biomarker that was elevated significantly in sputa from patients with COPD (Table 2) was evaluated within the patients stratified by GOLD status. Sialic acid was elevated in all GOLD groups relative to nonsmoking healthy control participants (Fig 1A), with a 2.8-fold (0.44-log) increase in GOLD 2 stage and 3.7-fold (0.56-log) increase in GOLD 3 stage relative to nonsmokers. Sialic acid also was elevated in the most severe disease cohorts (GOLD stages 2 and 3) relative to smokers with preserved spirometry and those with less severe disease (GOLD stage 1). Sialic acid has been identified as a biomarker of mucins,7 and sputum sialic acid concentrations were correlated significantly with previously reported total mucin concentration9 in the 748 samples in which both measures were available (e-Fig 2A).
Figure 1.
A-F, Box-and-whisker plots showing sputum biomarkers by patient group. A, Sputum sialic acid was increased in all GOLD stages relative to the NS group and increased in GOLD stage 2 and GOLD stage 3 groups relative to SPS or GOLD stage 1 groups. B, Adenosine metabolite hypoxanthine also was elevated in those with more severe disease (GOLD stages 2 and 3) relative to NS and SPS groups. C, Similar findings for xanthine as those in (B). D, E, Methionine salvage pathway metabolites MTA (D) and adenine (E) were elevated in GOLD stages 2 and 3 groups relative to the NS group. F, Differences in sputum GSH were modest among patient groups and were statistically significant only for the GOLD stage 2 group relative to the NS or SPS groups. ∗P < .05, ∗∗P < .01, or ∗∗∗P < .001 vs NS, respectively. ♦P < .05 or ♦♦P < .01 vs SPS group. ■■P < .01 vs GOLD stage 1 group. All P values from Tukey multiple comparisons test after analysis of variance. GOLD = Global Initiative on Chronic Obstructive Lung Disease; GSH = glutathione; MTA = methylthioadenosine; NS = nonsmoker; SPS = smokers with preserved spirometry.
To address the possibility that the sialic acid concentration findings reflected disease-mediated increases in sialic acid content of mucins (or other molecules) and not mucin concentration, we assessed the ratio of sialic acid concentrations to total mucins (as measured by refractometry). No differences in these parameters were detected among the study groups (e-Fig 2B). We also examined the ratio of sialic acid to urea, which we previously established as an index of mucus hydration.7 Sialic acid to urea ratio was elevated in all GOLD groups relative to nonsmokers (e-Fig 2C). Of note, sputum urea concentrations increased modestly but significantly with progressive disease (e-Fig 2D). Although this could reflect the known increase in serum urea in patients with COPD,11 serum urea measurements were not obtained in SPIROMICS.
The adenosine metabolites hypoxanthine and xanthine that were elevated in patients with COPD also were elevated in those with more severe disease by GOLD status relative to nonsmokers and smokers without obstruction (Fig 1B, 1C). No severity-related differences were observed in other metabolites within the adenosine metabolic pathway (adenosine, inosine), with the exception that adenosine was decreased in the cohort with the most severe (GOLD stage 3) disease relative to smokers without obstruction (e-Fig 3A-C).
Methylthioadenosine also was elevated in groups with GOLD stages 2 and 3 disease relative to nonsmokers, and within patients with GOLD stage 2 disease relative to smokers with preserved spirometry (Fig 1D). This metabolite is formed during synthesis of polyamines and is regenerated to methionine in the methionine salvage pathway. The enzymatic reactions in this pathway generate free adenine, which also was elevated in the most severe disease cohorts relative to nonsmokers (Fig 1E), although not elevated relative to smokers without obstruction. The polyamine spermine that was elevated in patients with COPD (although not significant by false discovery rate) (Table 2) did not vary by patient group (not shown).
Glutathione, which is linked closely to oxidative stress, was increased in patients with COPD, but statistically significant changes were observed only in the GOLD stage 2 group (Fig 1F) with modest changes over nonsmokers (1.7-fold, 0.23-log change). Of note, because all samples were processed with a strong reducing agent before analysis, measured concentrations reflect total glutathione (reduced plus oxidized), and not the oxidative state of the airway.
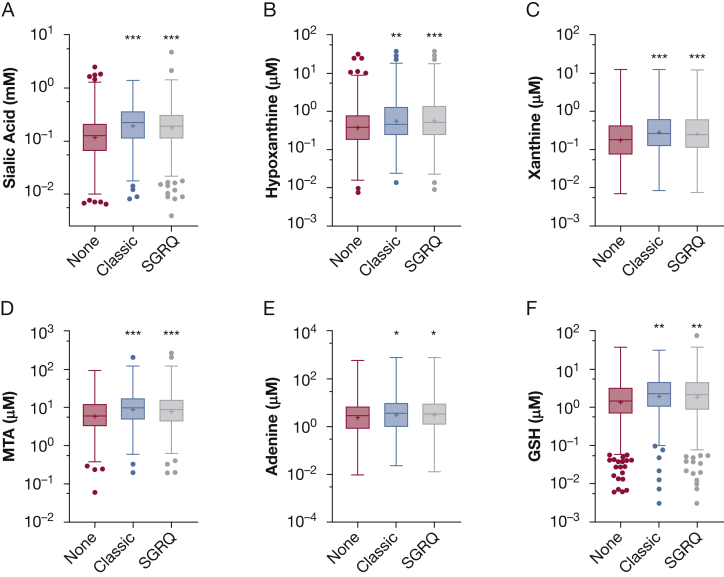
Sputum Biomarkers and Bronchitis
Because the biomarker panel included metabolites related to mucin secretion and inflammation, we predicted that measured biomarkers would be elevated in patients who reported clinical feature of bronchitis.2,9,12 In patients with chronic bronchitis assessed using either classic or St. George’s Respiratory Questionnaire definitions,10 sputum sialic acid was elevated significantly relative to sputum from patients with negative findings by both definitions (Fig 2A). Similar patterns were observed for the other biomarkers including hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione (Fig 2B-F).
Figure 2.
A-F, Box-and-whisker plots showing sputum biomarkers and bronchitis. A, Sputum sialic acid was increased in patients with chronic bronchitis defined by classic or SGRQ criteria vs those without chronic bronchitis. B-F, Similar findings were observed for hypoxanthine (B), xanthine (C), MTA (D), adenine (E), and GSH (F). ∗P < .05, ∗∗P < .01, or ∗∗∗P < .001 vs none, respectively. P values from Tukey multiple comparisons test after analysis of variance. SGRQ = St. George’s Respiratory Questionnaire; GSH = glutathione; MTA = methylthioadenosine.
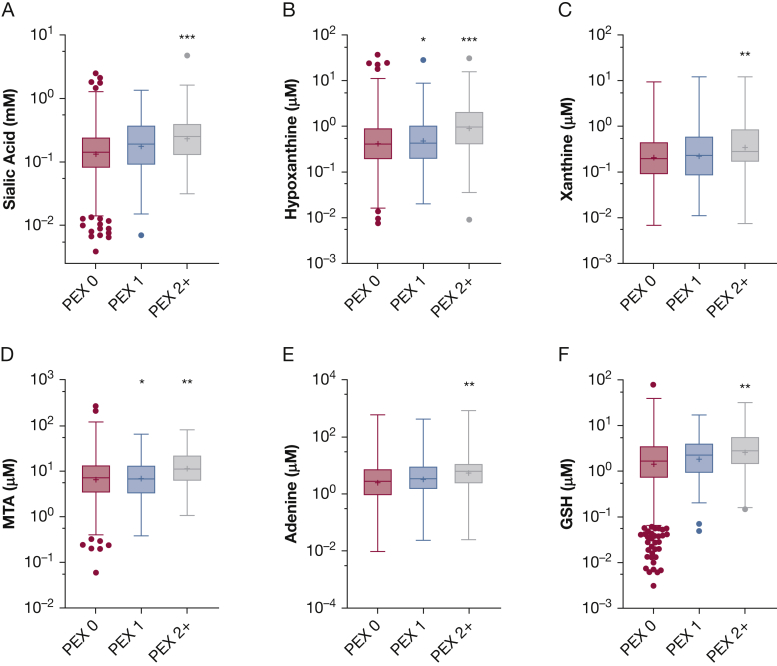
Sputum Biomarkers and Pulmonary Exacerbation
Because mucin secretion and inflammation also are related to the pathophysiologic characteristics of pulmonary exacerbations, we hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid (Fig 3A) and hypoxanthine (Fig 3B) were elevated significantly in those who experienced multiple (two or more) pulmonary exacerbations relative to those who experienced none. Similar, although less significant, findings were observed for xanthine, methylthioadenosine, adenine, and glutathione (Fig 3C-F).
Figure 3.
A-F, Box-and-whisker plots showing sputum biomarkers and pulmonary exacerbations. A, Sputum sialic acid was elevated in patients who experienced PEX2+ in the year after study entry relative to those with PEX 0. B, Sputum hypoxanthine was elevated in those who experienced PEX 2+ as well as those who experienced PEX 1. C-F, Similar observations were made for xanthine (C), MTA (D), adenine (E), and GSH (F). ∗P < .05, ∗∗P < .01, or ∗∗∗P < .001 vs PEX 0, respectively. P values from Tukey multiple comparisons test after analysis of variance. GSH = glutathione; MTA = methylthioadenosine; PEX 0 = 0 pulmonary exacerbations; PEX 1 = one pulmonary exacerbation; PEX 2+ = two or more pulmonary exacerbations.
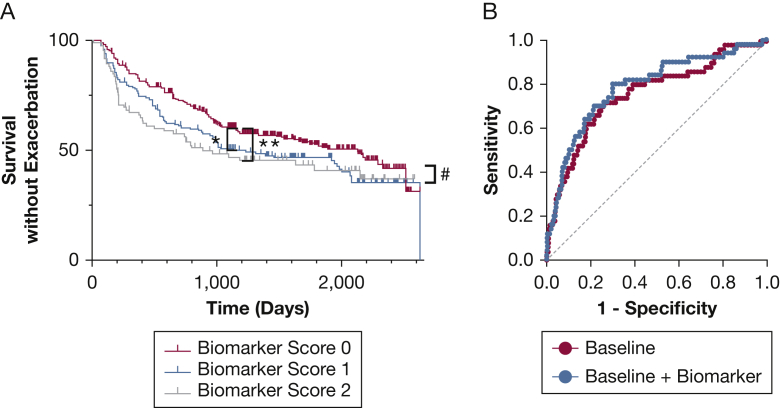
These relationships suggested that sputum biomarkers could be used to identify patients with COPD at higher risk for pulmonary exacerbations. Because sialic acid and hypoxanthine seemed to be the most predictive biomarkers, we developed a simplified biomarker score based on whether sialic acid, hypoxanthine, or both were elevated in sputum (see the Study Design and Methods section). In survival analysis, pulmonary exacerbations occurred earlier in patients with COPD (n = 562) with elevated sputum biomarker scores (Fig 4A). Within the entire dataset spanning up to 7 years, the cohort in which both sialic acid and hypoxanthine were elevated (biomarker score of 2) were statistically different from those with neither elevated (biomarker score of 0; P = .02 for the model by log-rank Mantel-Cox test, with P = .02 by log-rank after test after Tukey-Kramer adjustment). To examine shorter-term predictive ability, we repeated the analysis for data restricted to 3 years after the initial visit. In this model, both biomarker scores 1 and 2 were predictive of time to exacerbation (P = .008 for model, P = .027 for score 1, and P = .002 for score 2 after test). We then assessed whether these biomarkers could be used to predict which patients with COPD were at risk of multiple pulmonary exacerbations in year after the initial study visit. Using logistic regression, the biomarker score was modestly predictive of patients who experienced multiple pulmonary exacerbations (AUC, 0.664; 95% CI, 0.586-0.743; P < .001). We then assessed whether adding biomarkers could improve prediction of established models using four commonly used and easily measurable predictors of future exacerbations: history of previous exacerbations, percent predicted FEV1, smoking, and age.13 Using logistic regression, the baseline model was highly significant (P < .0001) for predicting those with COPD at risk of multiple future exacerbations, with an AUC of 0.758 (95% CI, 0.683-0.834). Adding the biomarker score to the baseline model resulted in a statistically significant increase in the diagnostic accuracy of the model with an AUC of 0.785. (95% CI, 0.713-0.857; P < .001 vs the baseline model) (Fig 4B). To verify these findings, we performed cross-validation AUC analyses, which confirmed that the cross-validated AUC of the baseline plus biomarker model (0.756; 95% CI, 0.731-0.781) was significantly higher than the cross-validated AUC of the baseline model alone (0.732; 95% CI, 0.705-0.759; P < .01 vs. baseline plus biomarker by Wilcoxon matched pairs test).
Figure 4.
A, B, Predictive ability of biomarkers. A, Time to exacerbation based on biomarker score reflecting elevated sialic acid or hypoxanthine levels, or both in patients with COPD (n = 562). In the full data set, exacerbations occurred sooner in those with a biomarker score of 2 relative to those with a biomarker score of 0 (P = .02 for the model by log-rank Mantel-Cox test, with P = .02 by log-rank after test after Tukey-Kramer adjustment). Analyses also were performed with data censored at 3 years to assess shorter-term predictions, and in this model, both biomarker scores 1 and 2 were predictive of time to exacerbation (P = .008 for model, P = .027 for score 1, and P = .002 for score 2 in after test). ∗P < .05 and ∗∗P < .01 by after test for model censored at 3 years, and #P < .05 by after test for full model. B, Receiver operating characteristic (ROC) curves from logistic regression to predict those with multiple future exacerbations. The baseline model (red) used prior number of exacerbations, percent predicted FEV1, age, and sex as variables (area under the ROC curve [AUC], 0.758; 95% CI, 0.683-0.834; P < .001). The baseline plus biomarker model (blue) adding a biomarker score showed modest but significant improvement (AUC, 0.785; 95% CI, 0.713-0.857; P < .001; P = .0059 vs baseline model by likelihood ratio test). Differences were confirmed using cross-validated AUC analyses (see text).
Discussion
We identified sputum biomarkers that were elevated in the airways of patients with COPD and showed significant relationships to established measures of COPD disease severity, including airway neutrophilic inflammation, GOLD status, bronchitis, and pulmonary exacerbations. The identities of these and related metabolites implicate several physiologic pathways in COPD pathogenesis, including mucus hydration, adenosine metabolism, and methionine salvage. These pathways can serve as both biomarkers and therapeutic targets in COPD.
Our data further demonstrate that hydrolyzed sialic acid can serve as a biomarker of airway mucins.7 Indeed, the 2-fold to 3-fold increases in sialic acid we observed within those with COPD of greater spirometric severity are consistent with prior studies measuring total mucin concentrations using physical techniques,9,14 and relationships to bronchitis and pulmonary exacerbations reported herein for sialic acid are nearly identical to those reported previously for total mucins.9 Importantly, this magnitude of mucus hyperconcentration is in a range predicted to cause osmotically induced slowing of mucus clearance, mucus stasis and accumulation, and ultimately, airflow obstruction, inflammation, and infection.15,16 Our data showing positive correlations between sialic acid and GOLD status demonstrate a relationship between mucus concentration and airflow obstruction, and the correlation between sialic acid and neutrophil numbers support a role for mucus stasis and accumulation in perpetuating airway inflammation. Collectively, our findings support the hypothesis that improving airway mucus hydration would have a beneficial effect on COPD outcomes.
The increases in hypoxanthine and xanthine associated with disease severity suggest that COPD is characterized by disease-mediated increases in the metabolism of extracellular airway adenosine, a finding consistent with the observed pattern of other adenosine metabolites in this study. Increased airway adenosine metabolism has been observed in other airway inflammatory diseases5,6 and may contribute to previously observed increases in serum hypoxanthine in COPD and ARDS.16,17 Elevated concentrations of adenosine metabolites may alter the pathophysiologic features of airways directly, because both hypoxanthine and xanthine are substrates for xanthine oxidase, which contributes to oxidative stress.17 Increased airway oxidative stress also could underlie the increased concentrations of glutathione seen in this study and others.3,18 However, we could not distinguish oxidized from reduced glutathione in this study because samples were processed using a strong reducing agent.
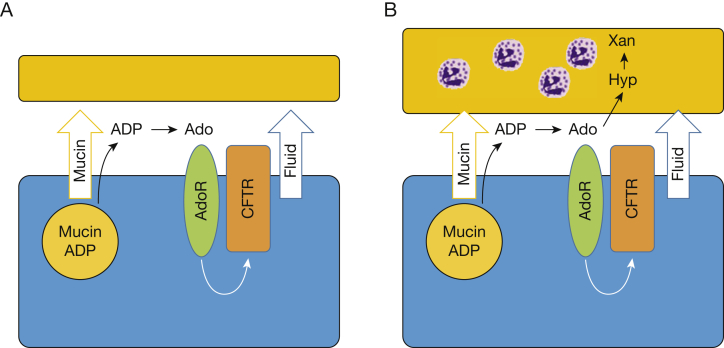
Increased airway adenosine metabolism also has implications for mucus hydration. Under normal circumstances, mucins and adenosine are released in a coordinated fashion in response to noxious stimuli such as cigarette smoke (Fig 5A), acting together to flush offending agents off airway surfaces and out of the lung.19 This coordinated release reflects copackaging of mucins with adenosine precursors (adenosine diphosphate, adenosine metabolic pathway) that are metabolized on airway surfaces to adenosine, as well as nucleotide release via other mechanisms.19, 20, 21 This balance of mucin and fluid secretion was observed in the smokers without COPD in whom increases in airway sialic acid (mucins) were coupled to increases in airway adenosine. However, our findings suggest that with disease progression (Fig 5B), increased mucin secretion rates were accompanied by increased extracellular adenosine metabolism, preventing sufficient adenosine-mediated mucus hydration. Thus, our findings are consistent with and provide a mechanistic explanation for the COPD severity-associated decreases in mucociliary clearance observed with prolonged smoking and airflow obstruction (COPD).22, 23, 24 The cause of the increased adenosine metabolic activity in COPD was not determined in this study, but could reflect epithelial cell reprograming or the action of airway macrophages or infiltrating neutrophils. The adenosine metabolites hypoxanthine and xanthine have been linked closely to neutrophilic inflammation in CF,5,6 and we found similar, although weaker, correlations in this study.
Figure 5.
A, B, Diagrams showing mechanisms of disease in COPD. A, In health, noxious stimuli such as smoking trigger release of mucin granules from airway epithelia into the extracellular space. These granules contain ADP and other nucleotides, which are metabolized on the airway surface to Ado. Ado stimulates AdoRs, which in turn activate CFTR and other channels to promote fluid secretion. The coordinated secretion of mucins and fluid acts to flush noxious stimuli off of the airway surface. B, With progressive disease, mucin secretion is enhanced, but increased extracellular metabolism of Ado from neutrophils or other factors limits its ability to stimulate fluid secretion. The resulting imbalance between mucin and fluid secretion leads to mucus accumulation. Ado = adenosine; AdoR = adenosine receptor; ADP = adenosine diphosphate; CFTR = cystic fibrosis transmembrane conductance regulator; Hyp = hypoxanthine; Xan = xanthine.
The relationship between mucus secretion and adenosine metabolism likely underlies the predictive power of the biomarkers. Patients with elevations in both sialic acid and hypoxanthine were at the highest risk for future pulmonary exacerbations, and adding these biomarkers enhanced the predictive ability of established models. Although the improvement was modest, its magnitude was similar to that reported for other biomarkers,13 and the biomarker score predicted time to exacerbation similar to, if not better than, plasma fibrinogen as one of the most well-established COPD biomarkers.25 Biomarker measurements could be added readily into clinical platforms, because both sialic acid and hypoxanthine can be quantified using well-established, commercially available colorimetric or fluorometric assays26,27 in sputum collected and processed using standardized methods.1,28 These biomarkers also could have value as pharmacodynamic markers in clinical trials, because several for therapeutics are approved or in development that impact the identified pathways.29,30 However, further effort to define the reliability and variability of these biomarkers over repeated assessments would be needed before they could be used as outcome measures.
One novel observation was identification of a role for the methionine salvage pathway in COPD lung disease. Although this pathway primarily serves to recycle methylthioadenosine back to methionine, increased activity of this pathway has been linked to inflammation in diseases such as sepsis31,32 and colitis.33 Intracellular methylthioadenosine is antiinflammatory, acting to decrease synthesis of proinflammatory polyamines,34 to induce macrophage pyroptosis via stimulation of Caspase-1 activity,31 and to decrease release of tumor necrosis factor α and other inflammatory mediators33 likely via activation of adenosine receptors.35 The increases in airway methylthioadenosine with COPD and positive correlations with inflammation may reflect compensatory mechanisms to inflammation triggered by other stimuli. Alternatively, increases in extracellular methylthioadenosine are associated with decreases in intracellular concentrations in sepsis,32 and similar mechanisms could be promoting inflammation in COPD.
Interestingly, unlike in CF,5,6 none of the measured airway amino acid or dipeptide metabolites were elevated significantly in COPD after correction for multiple testing (Table 1). These metabolites are thought to reflect neutrophil-derived protease activity, and the differences may reflect the less intense neutrophilic inflammation in COPD relative to CF.6,36 These differences also may reflect a difference in anatomic compartments, because our previous studies in CF primarily analyzed BAL fluid.5,6
A limitation to this study is that we measured only one sample per patient, so we did not capture intrapatient variability in biomarker measurements. Sputum is a heterogenous sample, and such variability may have contributed to the lower correlation between sialic acid and mucins observed herein compared with prior observations.7 However, the large number of samples measured and the nearly identical relationships to all disease outcomes assessed increases our confidence that the measured sialic acid was an effective surrogate marker of mucin concentration. Another limitation is that this study contrasts with our previous report of increased airway adenosine in COPD based on analysis of exhaled breath condensate.37 The reasons for this discrepancy are unclear, although it is possible that exhaled breath condensate samples different portions of the airway than sputum38,39 or that enzymatic activity within samples5,14 altered adenosine pathway metabolites after collection. Interestingly, we observed similar incongruities in CF, with airway inflammatory markers positively correlated with adenosine in exhaled breath condensate,40 but negatively correlated with adenosine in BAL fluid.6 Further investigation is needed to understand these discrepancies among airway sample types.
Interpretation
We identified several physiologic pathways altered in the airways of patients with COPD and associated with markers of disease severity, with the strongest relationships to metabolite biomarkers of mucus hydration and adenosine metabolism. These findings confirm previously demonstrated associations in COPD and identify new pathways. Although further validation is required, measurement of these sputum metabolites could provide a straightforward means to assess disease severity, to identify those at risk for future exacerbations, and to measure the impact of novel therapeutics.
Acknowledgments
Author contributions: C. R. E. is responsible for the content of this manuscript. C. R. E. obtained funding, performed the analyses, wrote the manuscript, and is the guarantor of the manuscript. W. K. O., M. K., W. H. A., A. C., and R. C. B. assisted with data analysis and edited the manuscript. C. M. D., A. T. H., R. G. B., R. P. B., J. M. W., E. C. O., A. P. C., Y. T., V. K., L. M. P., C. B. C., M. K. H., Y. J. H., W. W. L., and J. L. C. contributed to SPIROMICS and edited the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: SPIROMICS is supplemented by contributions made through the Foundation for the National Institutes of Health and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, BoehringerIngelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi, Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan. R. G. B. reports receiving grants from the Alpha1 Foundation. R. P. B. reports grants and personal fees from Boehringer Ingelheim; personal fees from Mylan Pharmaceuticals; and personal fees from Theravance; grants, personal fees, and nonfinancial support from GSK. J. M. W. reports grants from Bayer, grants and other from GSK, other from Boehringer Ingelheim, grants and other from Mereo BioPharma, and other from PRA. V. K. reports personal fees from Medscape, personal fees from Gala Therapeutics, personal fees from ABIM Critical Care Testwriting Committee, personal fees from AstraZeneca, and personal fees from Boehringer Ingelheim. A. P. C. reports personal fees from GSK and nonfinancial support from VIDA. C. B. C. reports personal fees from PulmonX, other from GlaxoSmithKline, personal fees from NUVAIRA, and personal fees from MGC Diagnostics. M. K. H. reports personal fees from GSK, personal fees from BI, personal fees from AZ, other from Sunovion, other from Novartis, and personal fees from Merck. J. L. C. reports personal fees from AstraZeneca. R. C. B. reports receiving fees from Parion Sciences. All disclosures are outside of the submitted work. None declared (C. R. E., W. K. O., W. H. A., M. K., A. C., C. M. D., N. E. A., A. T. H., E. C. O., A. P. C., Y. T., L. M. P., Y. J. H., W. W. L.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗SPIROMICS Group Collaborators: Current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell, Jr., MD; Robert Paine III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN.
Other contributions: The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data are available at www.spiromics.org.
Additional information: The e-Figures are available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: This work was supported by SPIROMICS, which was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Grants HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C, U01 HL137880, and U24 HL141762]. C. R. E. was supported by the National Institutes of Health [Grants R01-HL136961-01S1 and P30-ES10126]. R. C. B. was supported by the National Institutes of Health [Grants UH3-HL123645, P01-HL108808, P30-DK065988, P01-HL110873, P50-HL107168, and R01-HL136961].
Contributor Information
Charles R. Esther, Jr., Email: charles_esther@med.unc.edu.
Subpopulations and Intermediate Outcome Measures in COPD Study:
Neil E. Alexis, Wayne H. Anderson, Mehrdad Arjomandi, Igor Barjaktarevic, R. Graham Barr, Lori A. Bateman, Surya P. Bhatt, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Brad Drummond, Christine M. Freeman, Craig Galban, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Yvonne Huang, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Jr., Robert Paine, III, Laura Paulin, Stephen P. Peters, Cheryl Pirozzi, Nirupama Putcha, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J. Michael Wells, Robert A. Wise, and Prescott G. Woodruff
Supplementary Data
References
- 1.Hastie A.T., Martinez F.J., Curtis J.L., et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paone G., Leone V., Conti V., et al. Blood and sputum biomarkers in COPD and asthma: a review. Eur Rev Med Pharmacol Sci. 2016;20(4):698–708. [PubMed] [Google Scholar]
- 3.Beeh K.M., Beier J., Koppenhoefer N., Buhl R. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest. 2004;126(4):1116–1122. doi: 10.1378/chest.126.4.1116. [DOI] [PubMed] [Google Scholar]
- 4.Kinnula V.L., Ilumets H., Myllarniemi M., Sovijarvi A., Rytila P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J. 2007;29(1):51–55. doi: 10.1183/09031936.00023606. [DOI] [PubMed] [Google Scholar]
- 5.Esther C.R., Jr., Turkovic L., Rosenow T., et al. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J. 2016;48(6):1612–1621. doi: 10.1183/13993003.00524-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esther C.R., Jr., Coakley R.D., Henderson A.G., Zhou Y.H., Wright F.A., Boucher R.C. Metabolomic evaluation of neutrophilic airway inflammation in cystic fibrosis. Chest. 2015;148(2):507–515. doi: 10.1378/chest.14-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esther C.R., Jr., Hill D.B., Button B., et al. Sialic acid-to-urea ratio as a measure of airway surface hydration. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L398–L404. doi: 10.1152/ajplung.00398.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couper D., LaVange L.M., Han M., et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesimer M., Ford A.A., Ceppe A., et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377(10):911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones P.W., Quirk F.H., Baveystock C.M. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33-27. [DOI] [PubMed] [Google Scholar]
- 11.Mapel D.W., Marton J.P. Prevalence of renal and hepatobiliary disease, laboratory abnormalities, and potentially toxic medication exposures among persons with COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:127–134. doi: 10.2147/COPD.S40123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh D., Edwards L., Tal-Singer R., Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra B., Gaveikaite V., Bianchi C., Puhan M.A. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26(143):160061. doi: 10.1183/16000617.0061-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson W.H., Coakley R.D., Button B., et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192(2):182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson A.G., Ehre C., Button B., et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Button B., Cai L.-H., Ehre C., et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan G.J., Lamb N.J., Tilley R., Evans T.W., Gutteridge J.M. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med. 1997;155(2):479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- 18.Turgut T., Ilhan N., Deveci F., Akpolat N., Erden E.S., Muz M.H. Glutathione and nitrite levels in induced sputum at COPD patients and healthy smokers. J Thorac Dis. 2014;6(6):765–771. doi: 10.3978/j.issn.2072-1439.2014.04.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarowski E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8(3):359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarowski E.R., Sesma J.I., Seminario L., Esther C.R., Jr., Kreda S.M. Nucleotide release by airway epithelia. Subcell Biochem. 2011;55:1–15. doi: 10.1007/978-94-007-1217-1_1. [DOI] [PubMed] [Google Scholar]
- 21.Kreda S.M., Okada S.F., van Heusden C.A., et al. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584(pt 1):245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin B.K., Ramirez O., Zayas J.G., Finegan B., King M. Respiratory mucus from asymptomatic smokers is better hydrated and more easily cleared by mucociliary action. Am Rev Respir Dis. 1992;145(3):545–547. doi: 10.1164/ajrccm/145.3.545. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Wang X., Brighton L., Hazucha M., Jaspers I., Carson J.L. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21(10):875–881. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smaldone G.C., Foster W.M., O’Riordan T.G., Messina M.S., Perry R.J., Langenback E.G. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103(5):1390–1396. doi: 10.1378/chest.103.5.1390. [DOI] [PubMed] [Google Scholar]
- 25.Mannino D.M., Tal-Singer R., Lomas D.A., et al. Plasma fibrinogen as a biomarker for mortality and hospitalized exacerbations in people with COPD. Chronic Obstr Pulm Dis. 2015;2(1):23–34. doi: 10.15326/jcopdf.2.1.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond K.S., Papermaster D.S. Fluorometric assay of sialic acid in the picomole range: a modification of the thiobarbituric acid assay. Anal Biochem. 1976;74(2):292–297. doi: 10.1016/0003-2697(76)90210-4. [DOI] [PubMed] [Google Scholar]
- 27.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234(8):1971–1975. [PubMed] [Google Scholar]
- 28.Sagel S.D. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Curr Opin Pulm Med. 2003;9(6):516–521. doi: 10.1097/00063198-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Fahmi A.N., Shehatou G.S., Shebl A.M., Salem H.A. Febuxostat protects rats against lipopolysaccharide-induced lung inflammation in a dose-dependent manner. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(3):269–278. doi: 10.1007/s00210-015-1202-6. [DOI] [PubMed] [Google Scholar]
- 30.Ehre C., Rushton Z.L., Wang B., et al. An improved inhaled mucolytic to treat airway muco-obstructive diseases. Am J Respir Crit Care Med. 2019;199(2):171–180. doi: 10.1164/rccm.201802-0245OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko D.C., Gamazon E.R., Shukla K.P., et al. Functional genetic screen of human diversity reveals that a methionine salvage enzyme regulates inflammatory cell death. Proc Natl Acad Sci U S A. 2012;109(35):E2343–E2352. doi: 10.1073/pnas.1206701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Ko E.R., Gilchrist J.J., et al. Human genetic and metabolite variation reveals that methylthioadenosine is a prognostic biomarker and an inflammatory regulator in sepsis. Sci Adv. 2017;3(3) doi: 10.1126/sciadv.1602096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benight N.M., Stoll B., Marini J.C., Burrin D.G. Preventative oral methylthioadenosine is anti-inflammatory and reduces DSS-induced colitis in mice. Am J Physiol. 2012;303(1):G71–G82. doi: 10.1152/ajpgi.00549.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauter M., Moffatt B., Saechao M.C., Hell R., Wirtz M. Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem J. 2013;451(2):145–154. doi: 10.1042/BJ20121744. [DOI] [PubMed] [Google Scholar]
- 35.Keyel P.A., Romero M., Wu W., et al. Methylthioadenosine reprograms macrophage activation through adenosine receptor stimulation. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eickmeier O., Huebner M., Herrmann E., et al. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine. 2010;50(2):152–157. doi: 10.1016/j.cyto.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Esther C.R., Jr., Lazaar A.L., Bordonali E., Qaqish B., Boucher R.C. Elevated airway purines in COPD. Chest. 2011;140(4):954–960. doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabian P., Brain J., Houseman E.A., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24(3):137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexis N.E., Hu S.C., Zeman K., Alter T., Bennett W.D. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164(10 pt 1):1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 40.Esther C.R., Jr., Olsen B.M., Lin F.C., Fine J., Boucher R.C. Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(7):L504–L509. doi: 10.1152/ajplung.00344.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.