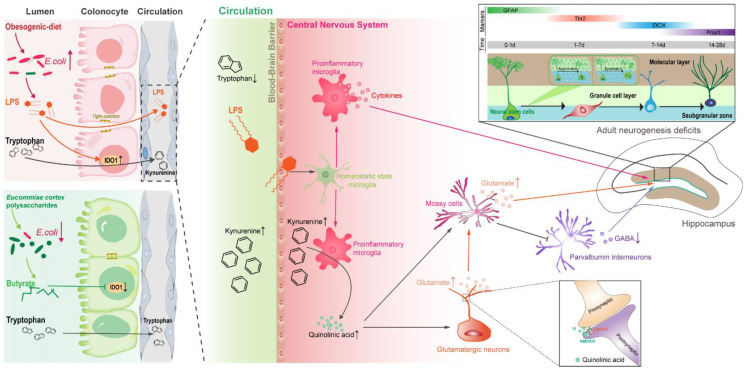
Figure 8.
Proposed mechanism for EPs mitigated OD-induced behavioral dysfunction. Persistent OD during adolescence reduced the abundance of SCFAs-producing bacteria but thrived facultative anaerobic bacteria, thereby increasing the endotoxin concentrations in colon lumen and circulation. In OD-fed mice, the IDO1 enzyme was activated in colonic cells, upregulating the peripheral Kyn pathway which promoted the production of Kyn and its downstream metabolites, including QA and Kna in the blood and brain parenchyma. Furthermore, infiltrating endotoxin stimulated the microglia toward proinflammatory phenotype which directs the metabolism of Kyn toward QA and increased extracellular glutamate levels in the hippocampus. QA-induced imbalance of GABAergic and glutamatergic transmission and microbiota-derived LPS induced neuroinflammation led to adult neurogenesis defects in the hippocampus and impaired hippocampus-dependent social and cognitive function in adolescent mice. EPs supplementation promoted the growth of butyrate-producing microbes and the production of butyrate and inhibited the abundance of E.coli, thereby alleviating OD-induced gut dysbiosis and peripheral Kyn pathway. This contributed to mitigating the QA-related neurotransmitter dysfunction and endotoxin-triggered neuroinflammation, thereby remodeling the rhythm of hippocampal neurogenesis and improving behavioral dysfunction in OD-fed mice.