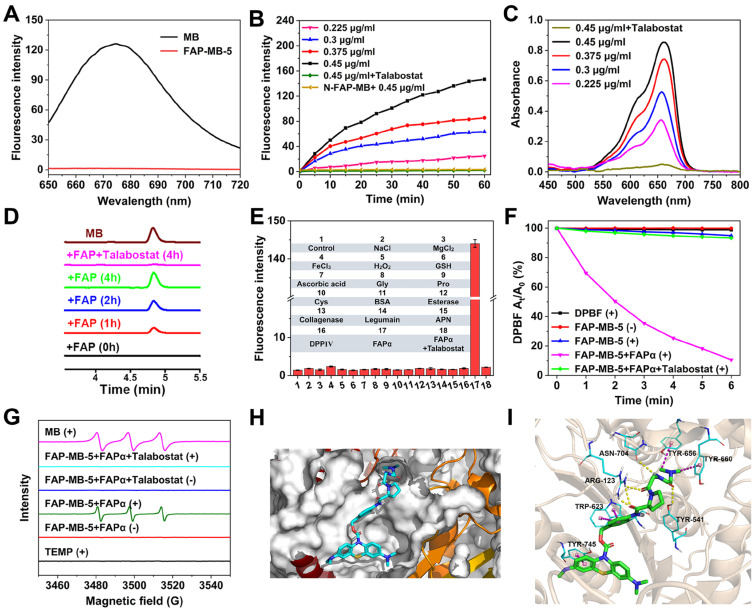
Figure 2.
(A) Optical characterization of fluorescence emission of FAP-MB-5 and MB in MeOH. (B) Fluorescence intensity of FAP-MB-5 (200 μM) pre-incubated with or without talabostat (100 μM) for 1 h towards various concentration of rhFAPα (0.225, 0.3, 0.375, 0.45 μg/mL) over time. Excitation: 630 nm. (C) UV-vis absorption spectra change of FAP-MB-5 (500 μM) towards various concentration of rhFAPα (0.225, 0.3, 0.375, 0.45 μg/mL). As inhibition group, rhFAPα (0.45 μg/mL) was pre-incubated with talabostat (100 μM) for 1 h, and then incubated with FAP-MB-5. (D) HPLC chromatogram of rhFAPα-mediated hydrolysis of FAP-MB-5 over time. As inhibition group, rhFAPα was pre-incubated with talabostat (100 μM) for 1 h. (E) Fluorescence response of FAP-MB-5 (200 μM) treated with the indicated protein (0.45 μg/mL unless otherwise specified), metal iron (50 μM) and other analytes (50 μM) for 1 h. 1, control; 2, Na+; 3, Mg2+; 4, Fe3+; 5, H2O2; 6, GSH; 7, ascorbic acid; 8, Gly; 9, Pro; 10, Cys; 11, BSA; 12, esterase; 13, collagenase; 14, Legumain; 15, APN; 16, DPPIV; 17, rhFAPα (0.225 μg/mL) pre-incubated with talabostat; 18, rhFAPα (0.225 μg/mL). (F) DPBF attenuation by 1O2 generation with different treatments in MeOH at 415 nm. (G) ESR spectra of different reaction systems with TEMP as the spin trap. (H) Docking analysis of the interactions of FAP-MB-5 with FAPα. (I) Detailed interactions between FAP-MB-5 and FAPα in a three-dimensional view. (+) and (-) refer to the treatment with or without irradiation, respectively. Results are described as mean ± SD, n = 3.