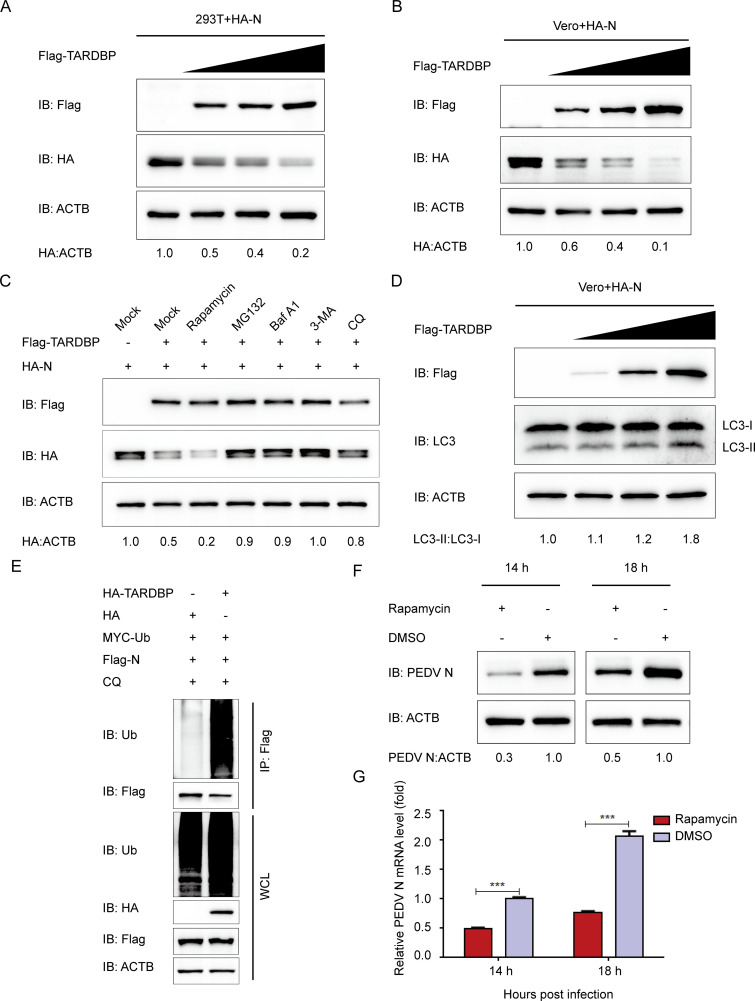
FIG 4.
TARDBP can degrade PEDV N protein by proteasomal and autophagic degradation. (A and B) A 24-h cotransfection of HEK 293T cells (A) and Vero cells (B) was accomplished using HA-N- and enhanced Flag-TARDBP-encoding plasmids. Subsequently, the cellular lysates were detected by Western blotting. (C) After transfection using HA-N- and Flag-TARDBP-encoding plasmids, the HEK 293T cells were subjected to separate processing with the rapamycin autophagy activator, the MG132 protease inhibitor, the Baf A1 (bafilomycin A1) autophagy inhibitor, 3MA (3-methyladenine), and CQ (chloroquine). Western blotting proceeded for investigating the cellular lysates. (D) An entire day of transfection of Vero cells was accomplished using the HA-N- and enhanced Flag-TARDBP-encoding plasmids. Subsequently, Western blotting proceeded for investigating the cellular lysates. (E) Cotransfection of HEK 293T cells with HA-TARDBP and Flag-N was accomplished, and then the cellular lysates were collected 24 h posttransfection. The ubiquitinated N proteins were immunoprecipitated with an anti-Flag antibody and explored by WB. (F and G) dimethyl sulfoxide (DMSO) or rapamycin was added to the Vero cells. Twenty-four hours later, PEDV infection of the cells was accomplished at an MOI of 0.01. At 14 and 18 h postinfection, the cellular lysate samples and supernatants were harvested for assessing the mRNA levels and expression of PEDV N protein independently via qRT-PCR and Western blotting.