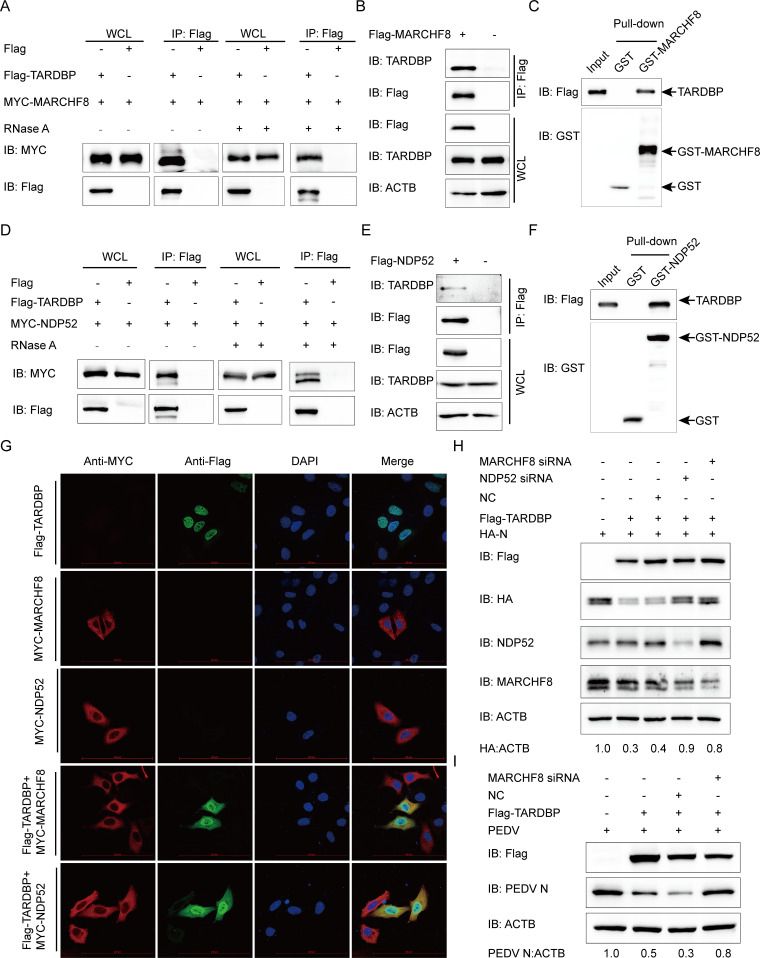
FIG 5.
TARDBP deteriorated N protein via the TARDBP-MARCHF8-NDP52-autophagosome axis. (A) Anti-Flag binding beads were utilized for the co-IP procedure before an entire day of transfection of HEK 293T cells using the Flag-TARDBP- and MYC-MARCHF8-encoding plasmids. The cells were collected and left untreated or treated with RNase. The precipitated proteins were analyzed by Western blotting. (B) Anti-Flag binding beads were utilized for the co-IP procedure, based on which an entire day of transfection of HEK 293T cells was accomplished using the Flag-MARCHF8-encoding plasmids. Western blotting was used to study precipitated proteins. (C) GST-MARCHF8 and TARDBP were detected by the GST affinity isolation assay. (D) A 24-h transfection of HEK 293T cells was accomplished using the Flag-TARDBP- and MYC-NDP52-encoding plasmids. The cells were collected and treated with RNase or left untreated. The precipitated proteins were analyzed by Western blotting. (E) An entire day of transfection of HEK 293T cells was accomplished using the Flag-NDP52-encoding plasmids prior to the co-IP procedure, where the anti-Flag binding beads were utilized. Subsequently, Western blotting proceeded for investigating the protein precipitates. (F) GST-NDP52 and TARDBP were detected via GST affinity isolation. (G) Flag-TARDBP and MYC-MARCHF8 or MYC-NDP52 were transfected into HeLa cells and subsequently labeled with antibodies, with cell nuclei labeled with DAPI, for confocal immunofluorescence microscopy. Scale bars, 100 μm. (H) HA-N, Flag-TARDBP, and siRNA (MARCHF8 siRNA, NDP52 siRNA, or NC siRNA) were cotransfected into HEK 293T cells. N protein abundance was detected via WB. (I) The Flag-TARDBP and MARCHF8 siRNA/NC siRNA were transfected into the Vero cells. PEDV infection of the cells 24 h later was accomplished at an MOI of 0.01, followed by gathering the cellular lysates for the Western blot-based expression evaluation of the PEDV N protein.