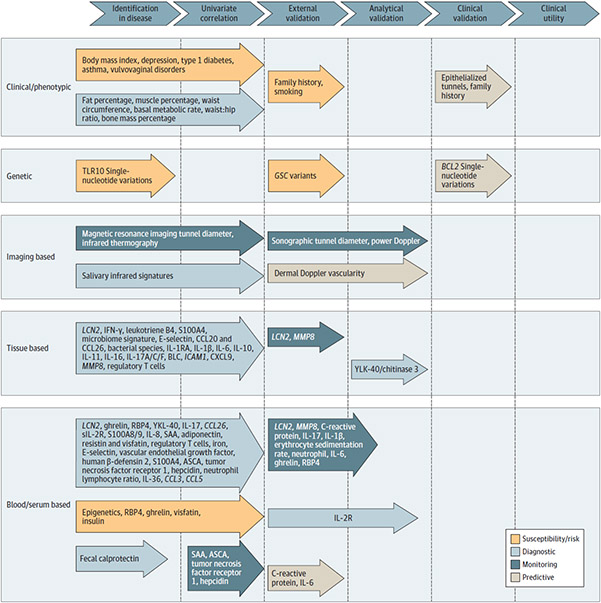
Figure. Biomarkers With GRADE Rating of Moderate or High and Degree of Biomarker Validation.
Multiple susceptibility/risk, diagnostic, and monitoring biomarkers were identified in this review, with lesser numbers of markers being independently validated in external cohorts. Predictive markers examining response to therapy were the only biomarkers that had undergone clinical validation in the setting of a clinical trial, and no biomarkers had assessment of clinical utility to recommend them for routine clinical use. The vast majority of these identified biomarkers met “moderate” GRADE criteria, and the only biomarkers that reached “high” GRADE criteria were serum IL-2R (diagnostic), dermal Doppler vascularity (monitoring), and epithelialized tunnels and positive family history of HS (predictive). Items were assessed based on criteria in line with the FDA biomarker definitions, GRADE criteria, and FDA/European Medicines Agency guidelines for the validation of proposed biomarkers as reported in eMethods in the Supplement. FDA indicates US Food and Drug Administration; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; GSC, gamma secretase complex; SAA, serum amyloid A.