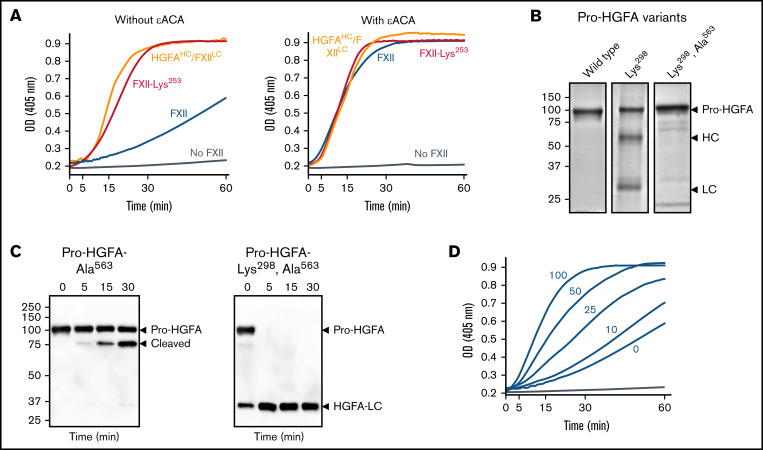
Figure 6.
Studies of the FXII KNG domain. (A) PK (60 nM) was incubated with S-2302 (200 μM) and 6 nM of FXII (blue), FXII-Lys253 (red), HGFAHC/FXIILC (orange), or vehicle (steel blue), without ε-ACA (left) or with 100 mM of ε-ACA (right) at 37°C. (B) Pro-HGFA Lys298 variants. Nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of purified wild-type Pro-HGFA (left), Pro-HGFA-Lys298 (center), and Pro-HGFA-Lys298 with the active site serine replaced with alanine (Pro-HGFA-Lys298, Ala563). Each lane was loaded with 2 μg of protein. Positions of markers for zymogen Pro-HGFA and the heavy and light chains of HGFA are shown to the right of the image. Positions of molecular mass standards in kilodaltons are shown to the left of the image. (C) Pro-HGFA activation in the absence of a surface. Pro-HGFA-Ala563 (left) or Pro-HGFA-Lys298, Ala563 (right), 260 nM each, were incubated with 26 nM of thrombin in the absence of a surface. At indicated times, samples were removed and size fractionated by reducing SDS-PAGE (12% polyacrylamide gel). Western blots of the gels were developed with an antibody that recognizes the C-terminal hemagglutinin tag added to the proteins. The antibody recognizes Pro-HGFA and HGFALC but will not recognize HGFAHC. The band marked cleaved in the left panel represents Pro-HGFA in which the N-terminal 88 amino acids (Gln1-Arg88) are removed by thrombin. This is not an active HGFA form. (D) Effect of ε-ACA on FXII/PK reciprocal activation. PK (60 nM) was incubated with 6 nM of FXII, S-2302 (200 μM), and varying concentrations of ε-ACA (10-100 mM) in Reaction Buffer at 37°C. For panels A and D, changes in optical density (OD) of 405 nm were continuously monitored on a spectrophotometer.