Figure 3.
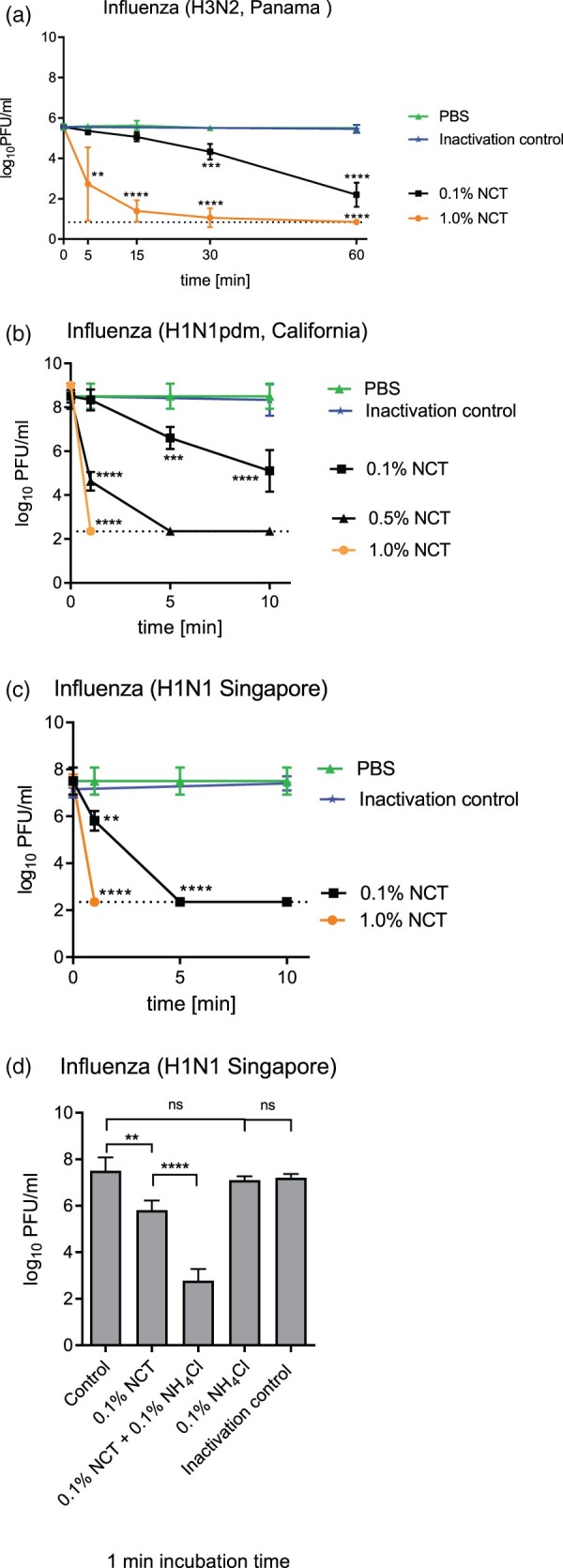
Inactivation of influenza viruses by NCT. a, Inactivation of Influenza A/Panama/2007/1999 (H3N2) by 1.0% (55 mM) and 0.1% (5.5 mM) NCT. Virus suspension was incubated with NCT or PBS for 5, 15, 30 min, or 60 min at 37°C, after which samples were diluted 1:1 in met/his solution for inactivation of NCT. Remaining infectious virus particles were determined using plaque titration. To control for inactivation of NCT by met/his, virus was added after dilution of 1.0% NCT in met/his in the inactivation control. Mean values ± SD of five independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus PBS control. Detection limit 0.84 log10 (dotted line). b, Inactivation of Influenza A/California/ Swine Origin Virus/2009 (H1N1pdm) by 0.1%, 0.5% and 1.0% NCT. Virus suspension was incubated with NCT in RPMI or plain RPMI for 1, 5, and 10 min at 22°C, after which samples were diluted 1:1 in met/his solution for inactivation of NCT. Remaining infectious virus particles were determined using plaque titration. Mean values ± SD of four independent experiments. Detection limit 2.35 log10 (dotted line). c, Inactivation of Influenza A/Singapore/Hongkong/2339/2000 (H1N1) by 0.1% and 1.0% NCT and (d) by 0.1% NCT and 0.1% (5.5 mM) NCT plus 0.1% (18.7 mM) ammonium chloride compared. Test procedure and number of independent experiments as in Figure 3b. Inactivation control in (d) consisting of 0.1% NCT plus 0.1% NH4Cl plus inactivator.
