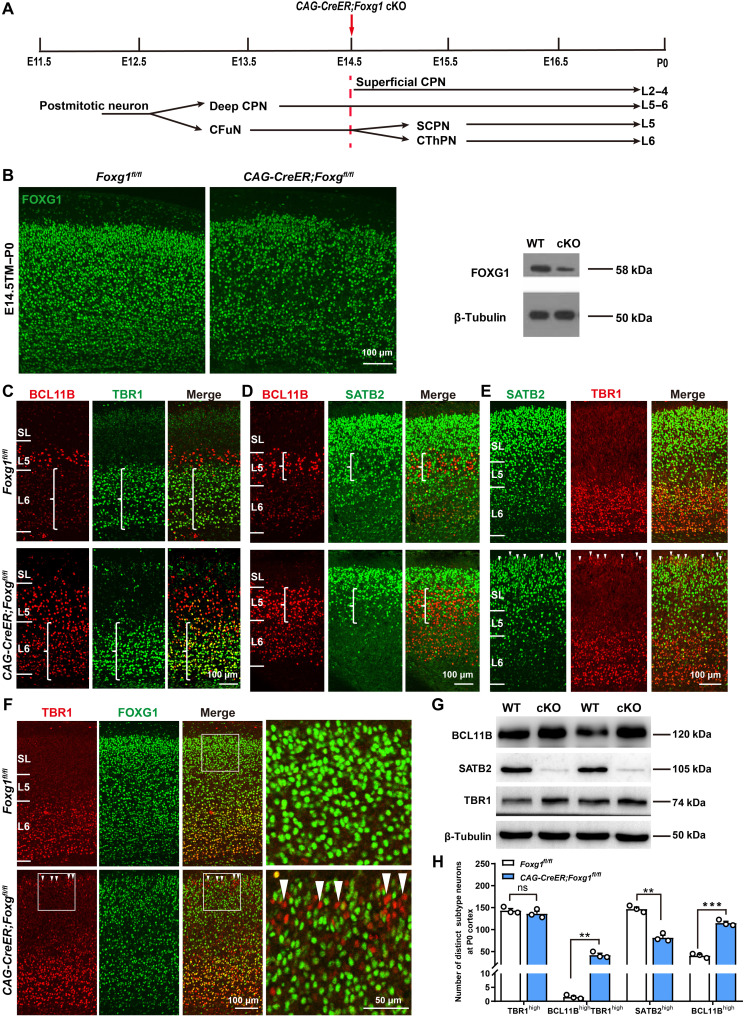
Fig. 2. Deletion of Foxg1 from E14.5 onward impairs the specification of CThPNs, deep CPNs, and superficial CPNs.
(A) Schematic of Foxg1 disruption strategy at E14.5. (B) Immunostaining and Western blot using P0 cortex, showing that Foxg1 was efficiently disrupted. (C to E) Double immunostaining at P0, showing failed BCL11B down-regulation in TBR1high CThPNs (C), decreased SATB2high deep CPNs and increased BCL11Bhigh neurons in L5 (D), and TBR1 up-regulation in SATB2low superficial CPNs in CAG-CreER;Foxg1fl/fl cKO cortices (E) (arrowheads). (F) Double immunostaining of TBR1 and FOXG1 at P0, showing up-regulation of TBR1 in FOXG1-deficient superficial CPNs (arrowheads). (G) Western blot of P0 cortex, showing increased levels of BCL11B and TBR1 and decreased levels of SATB2 in CAG-CreER;Foxg1 cKO mice. (H) Quantitative analysis showing an increase in BCL11BhighTBR1high and BCL11Bhigh neurons yet a reduction in SATB2high neurons in the deep layer at P0 in CAG-CreER;Foxg1 cKO mice. There were no obvious differences in the number of TBR1high neurons between control and CAG-CreER;Foxg1 cKO mice. Data are presented as means ± SEM; n = 3, multiple t test with Bonferroni correction. **P < 0.01; ***P < 0.001; ns, not significant. WT, wild type.