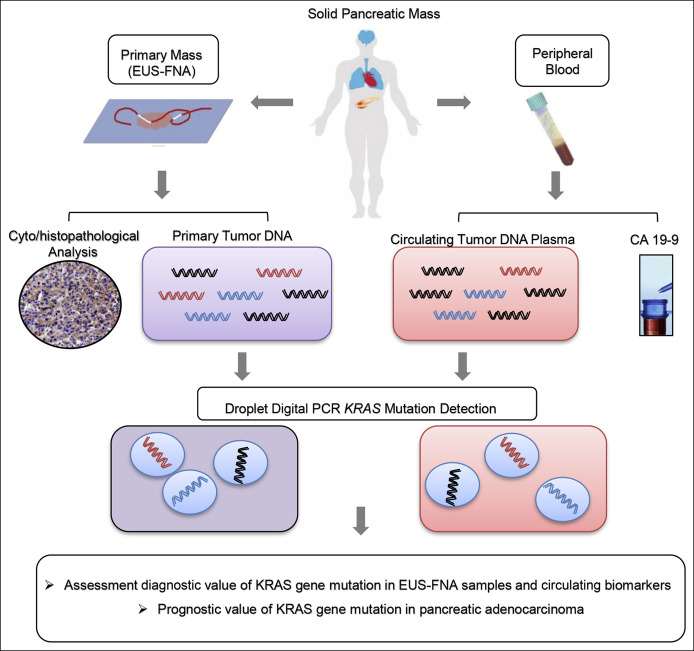
Figure 1.
Schematic diagram of digital PCR analysis for KRAS mutations in EUS-FNA specimens and ctDNA samples. ddPCR analyses were performed for tumor specimens obtained from patients with pancreatic cancer using either EUS-guided FNA cytology specimens or ctDNA from blood plasma. KRAS mutations were evaluated for potential clinical utility or as prognostic indicators. ctDNA, circulating tumor DNA; ddPCR, digital droplet PCR; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration.