We reported the preliminary results of the DisCoVeRy trial regarding the efficacy and safety of remdesivir in hospitalised patients with COVID-19 in February, 2022.1 Remdesivir did not have a clinical or virological benefit in the studied population. Notably, the number of patients included was lower than initially expected, because inclusions in this trial group were prematurely stopped by the data and safety monitoring board. Here, after completion of data monitoring, we report the final analysis, including two secondary endpoints that were not previously reported.
Briefly, the DisCoVeRy trial is a phase 3, open-label, randomised controlled trial evaluating the efficacy and safety of repurposed drugs in adults hospitalised for COVID-19, sponsored by Inserm (NCT04315948). Eligible participants were adults (aged ≥18 years) who were admitted to hospital with a positive PCR test for SARS-CoV-2 (<72 h before randomisation) and also had pulmonary rales or crackles with a peripheral oxygen saturation of 94% or less or required supplemental oxygen. The primary endpoint was the clinical status at day 15 as measured on a 7-point ordinal scale, which was analysed with a proportional odds model. Full details of the trial design are available in the preliminary report.1
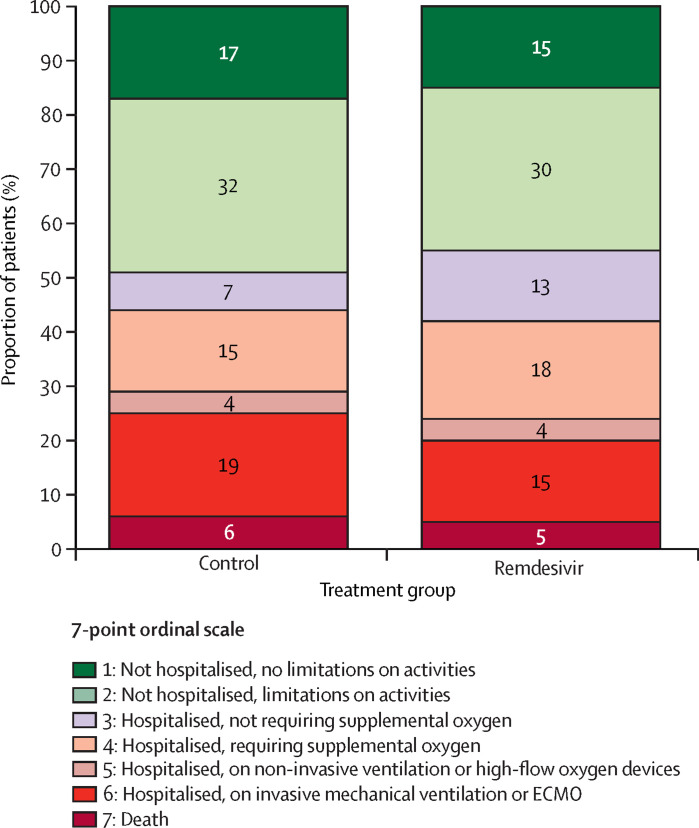
In the final dataset, 857 participants were randomly assigned to a treatment group and 843 participants (remdesivir, n=420; control, n=423) were evaluable for analysis. The final odds ratio (OR) for clinical improvement based on the primary endpoint and adjusted for disease severity at randomisation was not in favour of remdesivir (adjusted OR 1·02 [95% CI 0·62–1·70], p=0·93; figure ). This finding was consistent across all prespecified subgroup analyses.2
Figure.
Clinical status at day 15 of patients included in the intention-to-treat population
As measured by the 7-point ordinal scale. Reported numbers refer to the proportion of patients with the corresponding level in each group. The intention-to-treat population included all participants with a positive SARS-CoV-2 PCR test obtained in the past 9 days who were randomly assigned to a treatment group, for whom a valid consent form was obtained and who did not receive any investigational treatment in the past 29 days. ECMO=extracorporeal membrane oxygenation.
Full results regarding secondary outcomes are available elsewhere.2 Two secondary endpoints were not previously reported: in-hospital mortality and mortality at 3 months after randomisation. Remdesivir did not have a significant effect on in-hospital mortality (33 of 420 participants in the remdesivir group vs 38 of 423 participants in the control group; adjusted OR 0·84 [95% CI 0·51–1·37]; p=0·48), nor on mortality at 3 months (43 of 420 vs 49 of 423; 0·87 [0·56–1·36]; p=0·55). Similar to findings from preliminary analyses, participants from the remdesivir group who were not on mechanical ventilation or ECMO when they were randomly assigned to a treatment group (n=692) had a significantly longer time to the composite endpoint of new mechanical ventilation, ECMO, or death in the 29 days following randomisation than did the control group (cumulative incidence in the remdesivir group was 58 [17%] of 343 participants vs 88 [25%] of 349 in the control group; adjusted hazard ratio [HR] 0·63 [95% CI 0·45–0·88]; p=0·010). In non-prespecified analyses, this effect was significant in participants with severe disease when they were randomly assigned to a treatment group (cumulative incidence in the remdesivir group 25 [29%] of 87 vs 47 [50%] of 94 in the control group; unadjusted HR 0·49 [95% CI 0·30–0·80]; p=0·0040) but not in those with moderate disease (33 (13%) of 256 vs 41 (16%) of 255; 0·79 [0·50–1·25]; p=0·31). No significant effect of remdesivir on the viral kinetics was observed (effect of remdesivir on the slope of decrease of the nasopharyngeal viral load was –0·006 log10 copies per 10 000 cells per day [95% CI –0·02 to 0·03]; p=0·66).
Among the 833 participants included in the safety analysis (remdesivir, n=410; control, n=423), no significant difference was evidenced in the occurrence of grade 3–4 adverse events (143 of 410 participants in the remdesivir group vs 150 of 423 participants in the control group; unadjusted OR 0·98 [95% CI 0·73–1·32]; p=0·91) nor of serious adverse events (147 of 410 vs 138 of 423; 1·17 [0·87–1·57]; p=0·29).
Overall, the final results of the DisCoVeRy trial for the efficacy and safety of remdesivir reinforce the observations in the preliminary report, supporting recommendations against its use in hospitalised patients with COVID-19.
FM reports grants from INSERM Reacting (French Government), the Ministry of Health (French Government), and the European Commission, during the conduct of the study, and grants from Sanofi and Roche, unrelated to this Correspondence. MH reports grants from The Belgian Center for Knowledge and Fonds Erasme-COVID-ULB, during the conduct of the study, and personal fees from Gilead, unrelated to this Correspondence. CB reports personal fees from Da Volterra and Mylan Pharmaceuticals, unrelated to this Correspondence. All other authors declare no competing interests. This study received funding from the EU's Horizon 2020 Research and Innovation Programme, Austrian Group Medical Tumor, Belgian Health Care Knowledge Centre, Fonds Erasme-COVID-Université Libre de Bruxelles, Inserm REACTing network, the French Ministry of Health, Paris Ile-de-France Region, European Regional Development Fund, Portugal Ministry of Health, Portugal Agency for Clinical Research and Biomedical Innovation, European Union Commission, and Domaine d’intérêt majeur One Health Île-de-France. Remdesivir was provided by Gilead free of charge. FM and CB are joint last authors. Members of the DisCoVeRy Study Group are listed in the appendix.
Contributor Information
DisCoVeRy Study Group:
Jérôme Aboab, Florence Ader, Hafid Ait-Oufella, Antoine Altdorfer, Claire Andrejak, Pascal Andreu, Laurent Argaud, Firouzé Bani-Sadr, Drifa Belhadi, Leila Belkhir, François Benezit, Marc Berna, Mathieu Blot, Elisabeth Botelho-Nevers, Lila Bouadma, Olivier Bouchaud, David Bougon, Kevin Bouiller, Fanny Bounes-Vardon, Maude Bouscambert-Duchamp, David Boutoille, Alexandre Boyer, Sandra Braz, Cédric Bruel, Charles Burdet, André Cabié, Emmanuel Canet, Charles Cazanave, Cyrille Chabartier, Catherine Chirouze, Raphaël Clere-Jehl, Dominique Costagliola, Sandrine Couffin-Cadièrgues, Johan Courjon, Flora Crockett, François Danion, Aline Dechanet, Agathe Delbove, Jean Dellamonica, Christelle Delmas, Alpha Diallo, Félix Djossou, Clément Dubost, Alexandre Duvignaud, Alexander Egle, Olivier Epaulard, Loïc Epelboin, Hélène Esperou, Murielle Fartoukh, Karine Faure, Emmanuel Faure, Joao-Miguel Ferreira Ribeiro, Tristan Ferry, Cécile Ficko, Samy Figueiredo, Claire Fougerou, Vincent Fraipont, Benjamin Gaborit, Rostane Gaci, Amandine Gagneux-Brunon, Sébastien Gallien, Denis Garot, Alexandre Gaymard, Guillaume Geri, Sébastien Gibot, François Goehringer, Marie Gousseff, Richard Greil, Didier Gruson, Jérémie Guedj, Yves Hansmann, Olivier Hinschberger, Maya Hites, Stéphane Jaureguiberry, Vanessa Jeanmichel, Michael Joannidis, Solen Kerneis, Antoine Kimmoun, Kada Klouche, Marie Lachâtre, Karine Lacombe, Fabrice Laine, Bernd Lamprecht, Jean-Philippe Lanoix, Odile Launay, Bruno Laviolle, Minh-Patrick Lê, Vincent Le Moing, Jérôme Le Pavec, Yves Le Tulzo, Paul Le Turnier, David Lebeaux, Benjamin Lefevre, Sylvie Leroy, François-Xavier Lescure, Henry Lessire, Benjamin Leveau, Bruno Lina, Paul Loubet, Alain Makinson, Denis Malvy, Charles-Hugo Marquette, Guillaume Martin-Blondel, Martin Martinot, Julien Mayaux, Armand Mekontso-Dessap, France Mentré, Noémie Mercier, Ferhat Meziani, Jean-Paul Mira, Jean-Michel Molina, Xavier Monnet, Joy Mootien, Bruno Mourvillier, Bruno Mourvilliers, Marlène Murris-Espin, Jean-Christophe Navellou, Marion Noret, Saad Nseir, Walid Oulehri, José-Artur Paiva, Nathan Peiffer-Smadja, Thomas Perpoint, Gilles Peytavin, Gilles Pialoux, Benoît Pilmis, Vincent Piriou, Lionel Piroth, Julien Poissy, Valérie Pourcher, Jean-Pierre Quenot, François Raffi, Jean Reignier, Jean Reuter, Matthieu Revest, Jean-Christophe Richard, Béatrice Riu-Poulenc, Céline Robert, Pierre-Alexandre Roger, Claire Roger, Roberto Roncon-Albuquerque, Elisabeth Rouveix-Nordon, Yvon Ruch, Nadia Saidani, Juliette Saillard, Naomi Sayre, Eric Senneville, Albert Sotto, Thérèse Staub, Francois Stefan, Charles Tacquard, Nicolas Terzi, Julien Textoris, Guillaume Thiery, Jean-François Timsit, Violaine Tolsma, Sarah Tubiana, Jean-Marie Turmel, Florent Valour, Fanny Vardon-Bounes, Priyanka Velou, Gil Verschelden, Florent Wallet, Guilhem Wattecamps, Yazdan Yazdanpanah, and Yoann Zerbib
Supplementary Material
References
- 1.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022;22:209–221. doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir for the treatment of hospitalised patients with COVID-19: final results from the DisCoVeRy randomised, controlled, open-label trial. medRxiv. 2022 doi: 10.1101/2022.03.30.22273206. published online April 12. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.