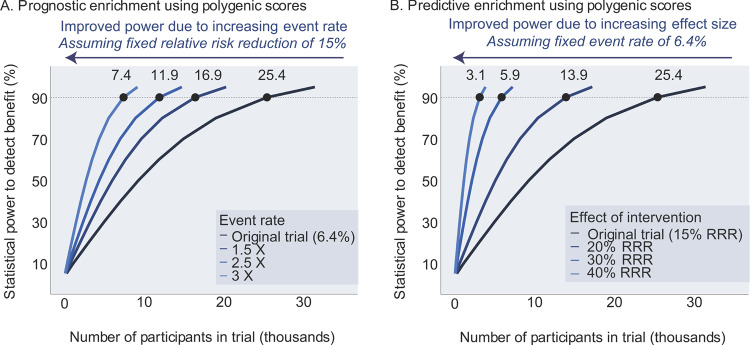
Fig. 1. Power and sample size estimation using prognostic or predictive model for polygenic score enrichment.
The FOURIER clinical trial randomized 27,564 patients with cardiovascular disease to a placebo or evolucumab, a cholesterol-lowering therapy, and followed patients for a median of 2.2 years4. This trial design was based on a power calculation that predicted an event rate of ~6.4% in the control arm and a relative risk reduction (RRR) of 15%28. We used these data to model power calculations using polygenic score enrichment under either of two models. A With prognostic enrichment (increasing event rates beyond the 6.4% in the original trial), a polygenic score enrichment improves statistical power to detect a benefit despite a fixed effect size (relative risk reduction of 15%); B with predictive enrichment (increasing effect size of intervention beyond the 15% RRR in the original trial), a polygenic score enrichment improves power with a fixed event rate in the placebo arm of 6.4%. The dashed line in both panels denotes 90% power to detect a statistical benefit, a threshold commonly used in trial design. Using polygenic scores to enrich clinical trials could markedly improve power and reduce the number of participants needed by increasing event rates (“prognostic enrichment”) and/or increasing the effect size (“predictive enrichment”).