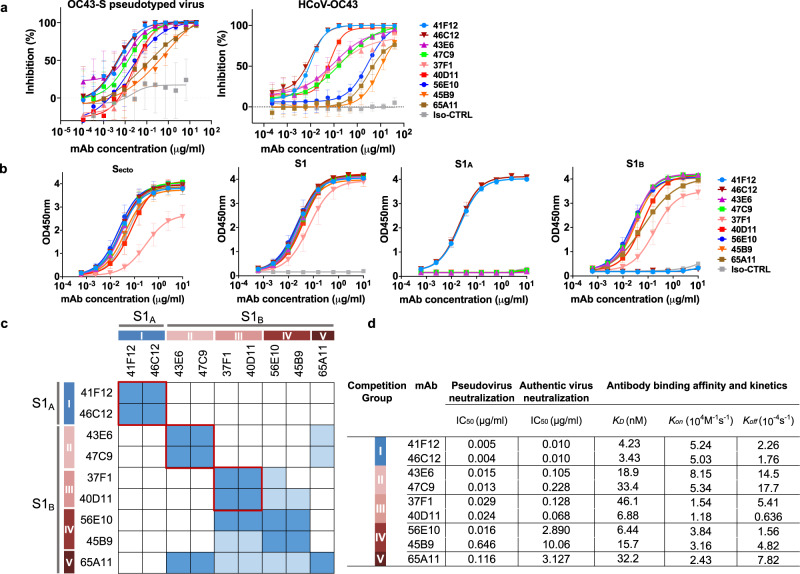
Fig. 2. Neutralizing and binding profile of OC43 S1-reactive human mAbs.
a Neutralizing activity of S1-directed human mAbs against OC43 S pseudotyped VSV (left panel) or authentic virus (USA/1967 ATCC strain, right panel) on HRT-18 cells. Data points represent means (±SD) of two independent experiments with six technical replicates. Iso-CTRL: antibody isotype control. b ELISA binding curves of mAbs to different types of OC43 S proteins. Data points represent means (±SD) of three independent experiments with each two technical replicates. Iso-CTRL: an anti-Strep-tag human monoclonal antibody was used as an antibody isotype control. c Heatmap showing binding competition of antibody pairs to the OC43 S1 protein, as determined by biolayer interferometry. Results are classified using color shading codes with a percentage of inhibition ≥75% in blue, <75% but ≥40% in light blue, and no shading for a percentage of inhibition <40%. Among the S1-binding antibodies, three mutually exclusive antibody groups (red rectangles, group I-III) were defined as well as two groups (IV and V) with a more miscellaneous binding competition profile. BLI sensorgrams showing the mAb binding competition profiles are shown in Supplementary Fig. 2. Each competition was performed twice independently, data from one representative experiment is shown. d Neutralizing potency and binding affinity of OC43 S1-directed human mAbs. Neutralization titers (IC50) were calculated based on inhibition curves shown in panel a. Antibody binding parameters determined by biolayer interferometry. BLI sensorgrams showing the kinetics of mAb binding to monomeric OC43 S1 antigen are shown in Supplementary Fig. 3.