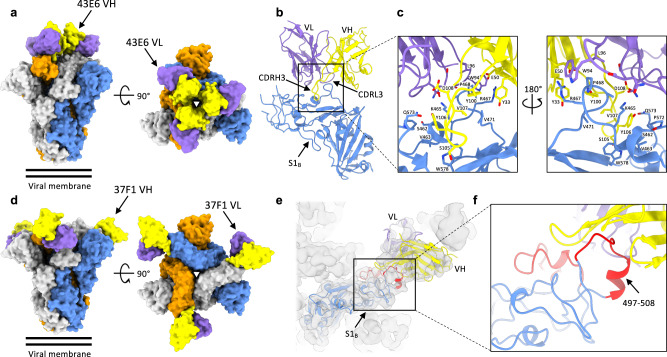
Fig. 4. Distinct, non-overlapping neutralizing epitopes in S1B identified by cryo-electron microscopy.
a Surface representation of the OC43 spike bound to three 43E6 antibody Fab fragments, shown as two orthogonal views. The threefold symmetry axis is denoted by a triangle. b Atomic model of a single OC43 S1B domain in complex with the 43E6 Fab fragment. c Zoomed-in view of the OC43 S 43E6 Fab binding site. d Surface representation of the OC43 spike bound to three 37F1 antibody Fab fragments, shown as two orthogonal views. e Homology model of a single OC43 S1B domain in complex with the 37F1 Fab fragment, fitted into the gaussian filtered EM density. The flexible loop regions that are not resolved in previous OC43 S structures are colored red. f Zoomed-in view of the OC43 S 37F1 Fab binding site. In (a–f), each OC43 S protomer is colored distinctly (gray, blue, and orange), whereas the heavy- and light-chain variable domains are colored yellow and purple, respectively.