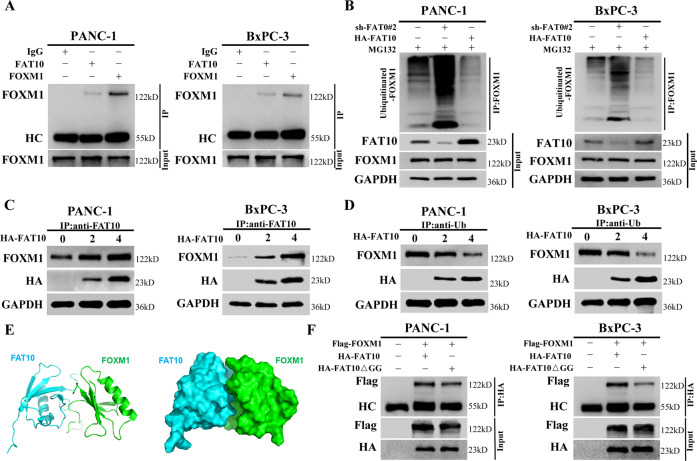
Fig. 7. FAT10 competes with ubiquitin to bind and stabilize FOXM1.
A Co-IP was used to detect the interaction between FOXM1 and FAT10 in PANC-1 and BxPC-3 cells. HC, heavy chain. B MG132 (10 μM) was added to PANC-1 and BxPC-3 cells, shFAT10 or HA-FAT10 plasmid was transfected at the same time, and then co-IP was used to detect the levels of ubiquitin bound to FOXM1 protein. C PANC-1 and BxPC-3 cells were transfected with different amounts of HA-FAT10 plasmid, and the level of FAT10 binding to FOXM1 protein was detected by co-IP. D PANC-1 and BxPC-3 cells were transfected with different amounts of HA-FAT10 plasmid, and the level of Ub binding to FOXM1 protein was detected by co-IP. E Docking conformation of the first ranking score. Three-dimensional structure of FAT10 and FOXM1. FAT10 is shown in green. FOXM1 is shown in cyan. F The Flag-FOXM1 plasmid was transfected into PANC-1 and BxPC-3 cells, the HA-FAT10 or HA-FAT10ΔGG plasmid was transfected at the same time, and then the protein level of Flag-FOXM1 bound to the HA-tagged protein was detected by co-IP. HC, heavy chain.