Figure 7.
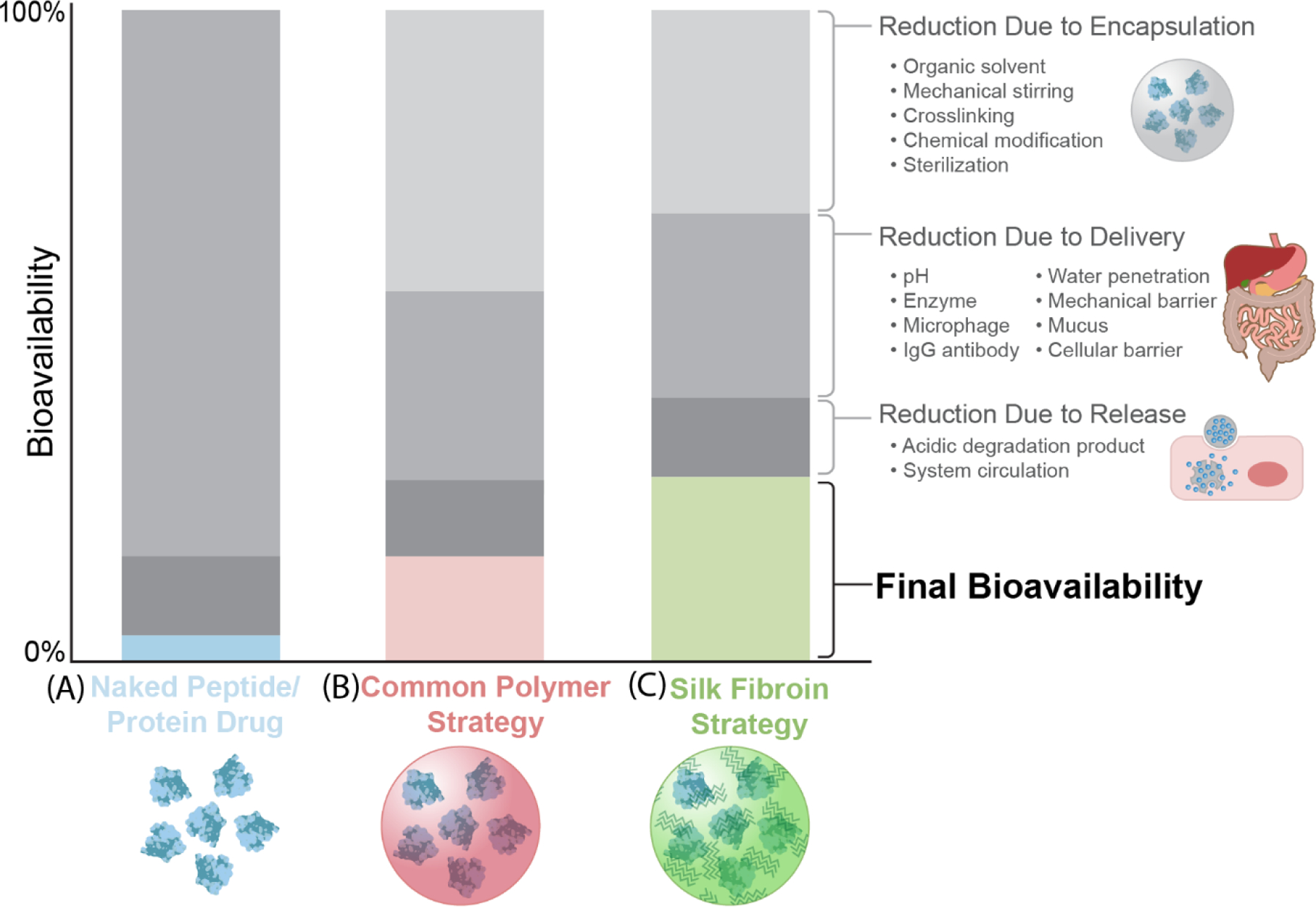
Comparison of bioavailability of the peptides/protein drugs without protection, with common synthetic polymer strategies, and with a silk protection strategy. A. The bioavailability of naked peptide/protein drugs affected by factors during encapsulation, delivery, and release. B. The bioavailability of peptide/protein drugs protected using common polymer strategies affected by factors during encapsulation, delivery, and release. C. The bioavailability of peptide/protein drugs protected using silk fibroin strategy affected by factors during encapsulation, delivery, and release. The decrease of the bioavailability in the figure is a qualitative analysis, to provide a visual comparison of the differences between the polymeric and silk strategies.
