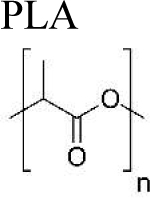
|
Encapsulation |
1. flexible to tune physical properties (size, shape) |
hydrophobic polymer: requires hydrophilic polymer to increase bioactivity of protein toxicity of solvent and stabilizers, emulsifiers, other additives high energy mechanical mixer or homogenizer needed high cost of particle production some sterilization methods require high temperature (glass transition temperature 40–60°C)
|
| Delivery |
FDA approved biocompatible low level of immunogenicity and toxicity
|
sensitivity to pH water penetration leads to denaturation of protein drugs surface modification important for eluding immune system uptake in reticuloendothelial system (RES) system not mucoadhesive
|
| Release |
rate of degradation dependent on crystallinity and polymer grafting selective release at high pH
|
acidic degradation products denature proteins
|
|
Encapsulation |
hydrophobicity and drug encapsulation tuned by glycolic and lactic acid content
|
solvent, stabilizers, emulsifiers, other additives toxicity concerns high energy mechanical mixing and homogenization low drug encapsulation capacity high cost of particle production sterilization thermal processing (glass transition temperature 40–60°C) not mucoadhesive initial burst release in acidic pH
|
| Delivery |
mechanical stability
|
surface functionalized of PLGA particles will compromise mechanical strength sensitivity to pH
|
| Release |
crystallinity tuned by copolymer composition
|
degradation products glycolic and lactic acids, decrease the bioactivity of protein drugs bulk erosion of polymer - entire volume, less controlled
|
|
Encapsulation |
chemical and thermal stability non-ionic surfactant as stabilizing agent, viscosity modifier excellent flow properties and high compressibility hydrophilic – higher encapsulation capacity
|
difficult to process as insoluble in most organic solvents and high viscosity soluble in water only when heated chemical/physical crosslinking required
|
| Delivery |
biocompatible
|
low stability in aqueous media particle swelling not mucoadhesive
|
| Release |
biodegradable
|
degradation product acidic, harmful for protein drugs less controlled release due to bulk erosion, swelling controlled release
|



