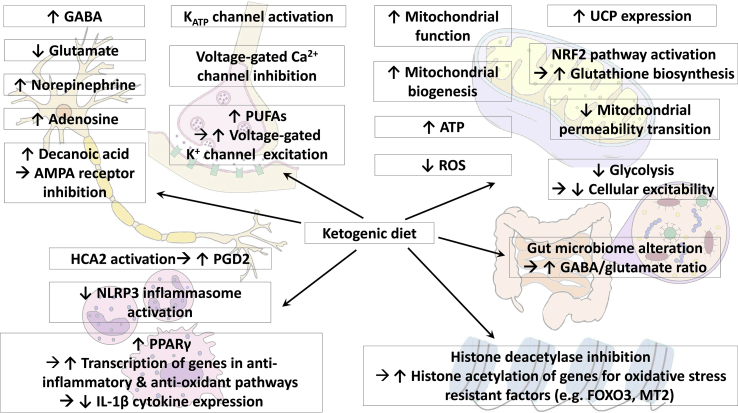
Fig. 1.
Proposed mechanisms of action of the KD. KD alters neurotransmitter levels: GABA, norepinephrine, and adenosine levels are increased, while glutamate level is decreased. Increased decanoic acid levels in MCT KD directly inhibits AMPA receptors. KD induces changes in regulation in ion channels: ATP-sensitive potassium channels are activated and voltage-gated calcium channels are inhibited. Increase in polyunsaturated fatty acids also results in increased excitation of voltage-gated potassium channels. KD enhances mitochondrial function and biogenesis, inducing increased ATP production and decreased ROS production. KD enhances expression of uncoupling proteins and increased glutathione biosynthesis via NRF2 pathway activation, both of which lead to decreased ROS production as well. KD inhibits mitochondria permeability transition, and thus inhibits apoptotic and necrotic cell death. Decreased glycolysis during KD decreases cellular excitability. KD activates hydroxy-carboxylic acid receptor 2, which in turn stimulates the synthesis of prostaglandin D2, inducing a neuroprotective phenotype in monocytes and/or macrophages. KD also suppresses NLRP3 inflammasome activation. KD increased PPARγ expression, which in turn diminishes IL-1β levels. KD increases histone acetylation by inhibiting histone deacetylases, leading to increased transcription of genes encoding for oxidative stress resistant factors (FOXO3 and MT2). KD induces gut microbiome alteration, which in turn leads to increased GABA/glutamate ratio. AMPA, alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid; ATP, adenosine triphosphate; FOXO3, forkhead box O3; GABA, gamma-aminobutyric acid; HCA2, hydroxy-carboxylic acid receptor 2; IL-1β, interleukin 1β; KD, ketogenic diet, MCT, medium-chain triglyceride; MT2, metallothionein 2; NRF2, nuclear factor E2-related factor 2; PGD2, prostaglandin D2; PPARγ, peroxisome proliferator activated receptor gamma; PUFAs, polyunsaturated fatty acids; ROS, reactive oxygen species; UCP, uncoupling protein.