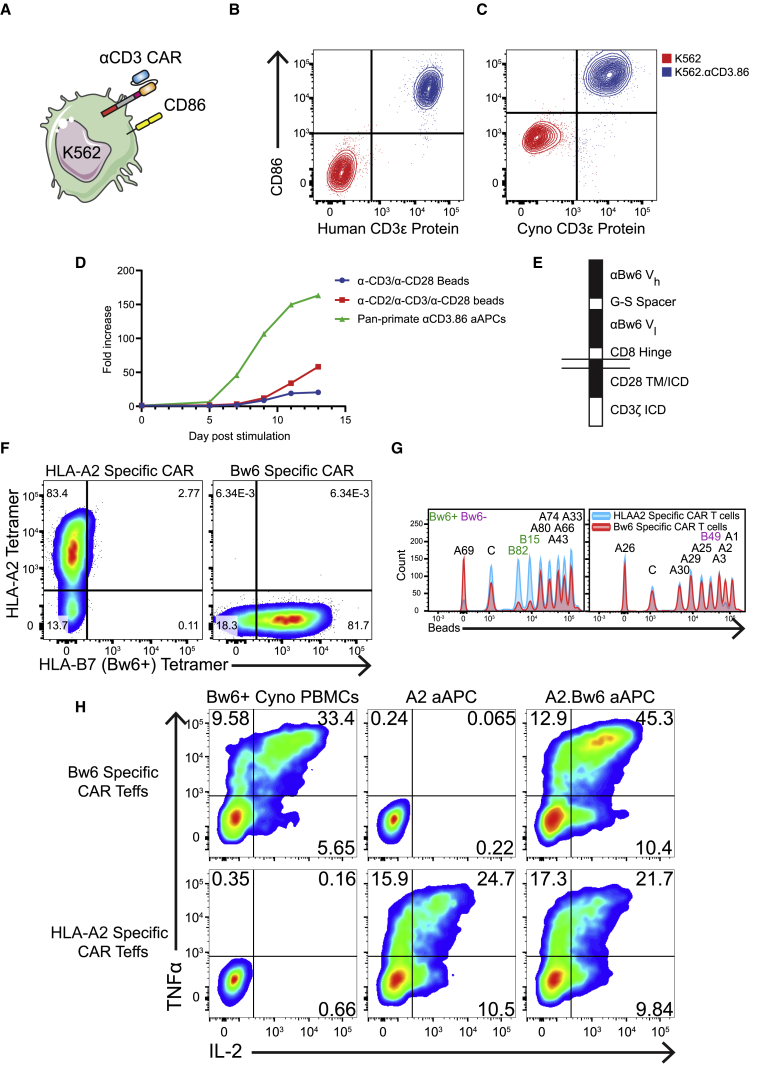
Figure 1.
Generation of Bw6-specific CAR Teffs with pan-primate α-CD3 aAPCs
(A) Cartoon of aAPCs (K562s) engineered to express αCD3 CAR and CD86.
(B and C) Parental and engineered K562 aAPCs were stained with biotinylated, recombinant human CD3ε protein plus streptavidin-PE (B) or with His-tagged Cynomolgus macaque CD3ε protein plus α-His antibody (C), followed by α-CD86 antibody.
(D) Growth curve of Teffs co-cultured with aAPCs expressing pan-primate αCD3 and human CD86, α-CD3/α-CD28-coated beads, or α-CD2/α-CD3/α-CD28-coated beads. Cells were counted every 2 to 3 days and diluted with media. Data are representative of two independent experiments.
(E) Schematic of Bw6-specific CAR. ICD, intracellular domain; TM, transmembrane domain; Vh, antibody variable heavy domain; Vl, antibody variable light domain.
(F) Cynomolgus macaque T cells were activated with aAPCs and transduced with χHIV lentiviral vectors encoding Bw6-specific CAR or HLAA2-specific CAR and then stained with both HLA-A2 and HLA-B7 (Bw6) tetramers.
(G) HLAA2-specific (blue) or Bw6-specific (red) human CAR T cells were incubated with single-antigen FlowPRA beads before analysis on a flow cytometer. Each peak represents beads conjugated to a unique HLA molecule (black, HLA-A molecules; green, Bw6+ HLA-B molecules; purple, Bw6− HLA-B molecules). Histograms are gated to depict unbound beads, such that a drop in frequency represents binding to CAR T cells. C, control beads.
(H) Human CAR Teffs were co-cultured for 5 h with the indicated target before staining with α-TNFα and α-IL-2 antibodies. Data are representative of three independent experiments.