Figure 2.
Generation and expansion of Cynomolgus macaque CAR Tregs in vitro
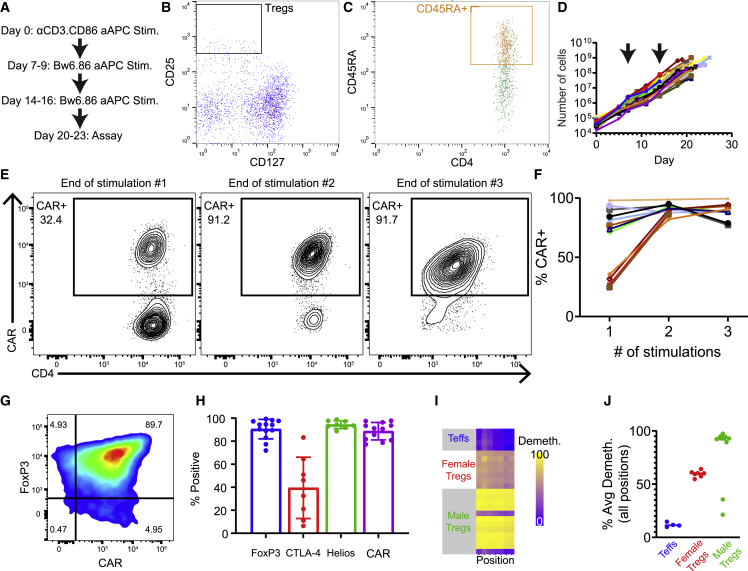
(A–C) Outline of Treg expansion protocol. Freshly isolated PBMCs from Cynomolgus macaque were stained with CD4, CD25, CD127, and CD45RA antibodies and flow sorted to obtain the top 1% to 2% of CD25hi CD127−/lo population (B) that are CD45RA+ (C).
(D) Following sort, irradiated α-CD3.CD86 aAPCs were co-cultured with Tregs at one aAPC per one Treg. After 48 h, Bw6-specific CAR lentiviral vector was added, and the scheme outlined in Figure 3A was followed to expand the Bw6-specific CAR Tregs. Cells were counted every 2 to 3 days, and cell growth was graphed, with each line representing 1 of 19 independent sorts. Arrows indicate days of restimulation with irradiated Bw6.86 aAPCs.
(E) Tregs were stained with HLA-B7 (Bw6+) tetramer upon resting before each restimulation. Displayed is a representative example showing CAR Treg enrichment after antigen-specific restimulation.
(F) Summary data from 12 experiments showing CAR Treg enrichment via restimulation. Each line represents one independent sort and expansion.
(G) At the end of expansion, cells were stained for FoxP3 and Bw6-specific CAR expression.
(H) Summary of 13 experiments showing the percentage of expanded Bw6-specific Tregs expressing FoxP3, CTLA-4, Helios, and Bw6-specific CAR at the conclusion of expansion. Data are represented as mean ± SEM.
(I) Genomic DNA collected at the end of cell culture was assessed for methylation of FOXP3 at the Treg-specific demethylated region (TSDR) by bisulfite sequencing. Each row represents one independent product, and each column represents one CpG locus in the TSDR.
(J) Summary of average TSDR demethylation across all loci from 18 experiments with six different animals. Each data point is one Treg expansion, and line represents the group mean.