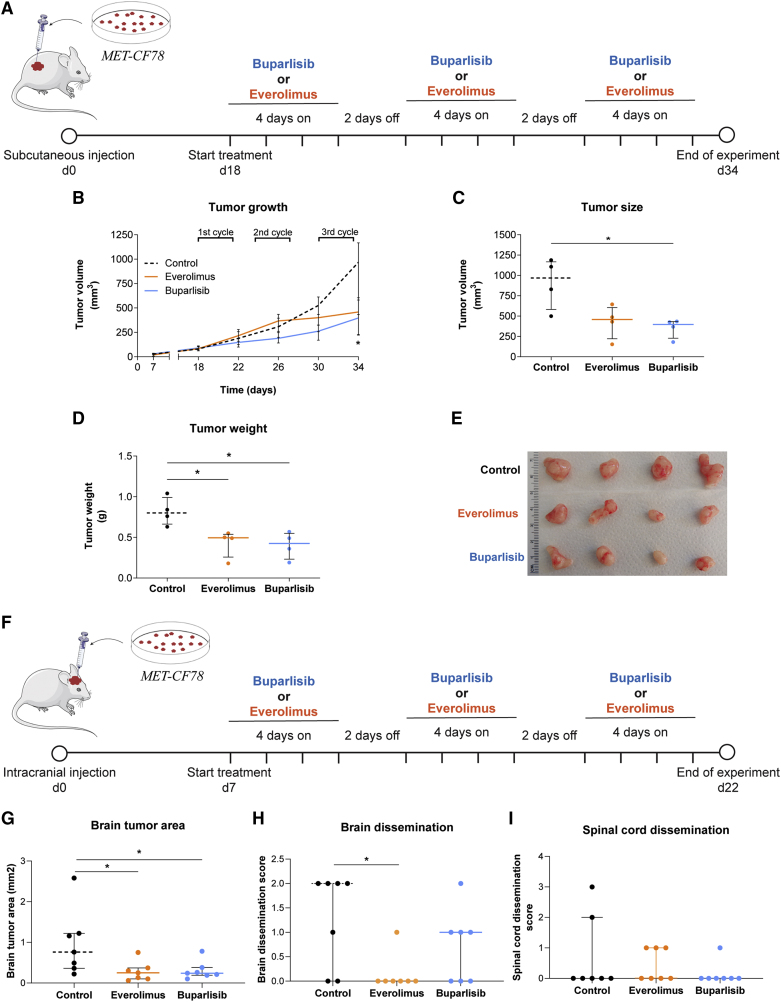
Figure 6.
PDXs of a lung cancer BM respond to PI3K and mTOR inhibition
(A) Representative scheme of the treatment protocol performed in a xenograft established after the subcutaneous injection of the PDC derived from a lung cancer BM (MET-CF78). Animals were randomly divided in three groups: buparlisib (30 mg/kg/day; n = 4), everolimus (3 mg/kg/day; n = 4), and vehicle (5% DMSO/30% PEG300/H2O; n = 4) used as control for comparison.
(B–D) Inhibition of flank tumor growth upon three cycles of therapy with buparlisib and everolimus (B). Significant reduction in (C) tumor size and (D) tumor weight by the end of treatment.
(E) Representative photographs of flank tumors in each experimental group by the end of treatment.
(F–I) Representative scheme of the treatment protocol performed in an orthotopic xenograft established after intracranial injection of the same PDC (F). Animals were randomly divided into three groups: buparlisib (30 mg/kg/day; n = 7), everolimus (3 mg/kg/day; n = 7), and vehicle (5% DMSO/30% PEG300/H2O; n = 7) used as control for comparison. Treatment administration was also performed in three cycles of therapy with buparlisib and everolimus. Histological sections of the CNS were evaluated to assess the (G) tumor area in the brain as well as (H) brain and (I) spinal cord dissemination. Data are represented as median with interquartile range. Differences were considered statistically significant for p values <0.05, according to the Mann-Whitney test. See also Figures S6 and S7.