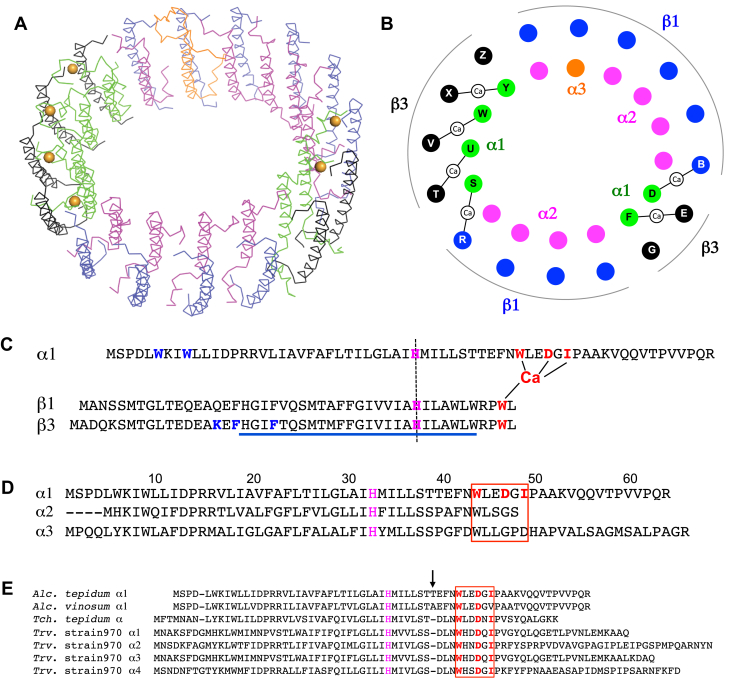
Figure 2.
Ca2+-binding motif and arrangement of the Allochromatium tepidum LH1 multiple polypeptides.A, tilted view of the LH1–RC from the periplasmic side of the membrane. Color scheme: α1, green; α2, magenta; α3, orange; β1, blue; β3, black; Ca2+, orange ball. B, illustration of the arrangement of the LH1 polypeptides. Letters in the colored circles denote chain IDs. Color scheme as in (A). C, sequence alignment showing the relative positions of the Ca2+- and BChl a-binding sites. The Ca2+-bound αβ-polypeptides are aligned relative to the BChl a-coordinating histidine residues (magenta letters with vertical dashed line). The Ca2+-ligating residues are shown in red letters. Key residues interacting in the N-terminal regions of α1- and β3-polypeptides are shown in blue letters. The underlined region represents a predicted membrane-spanning domain. D, sequence alignment of the α1-, α2-, and α3-polypeptides relative to the BChl a-coordinating histidine residues showing that the specific Ca2+-binding motif (WxxDxI) is only present in the α1-polypeptide. E, sequence alignment of the A. tepidum α1-polypeptide with those of A. vinosum, T. tepidum and Trv. strain 970 relative to the BChl a-coordinating histidine residues. The Ca2+-binding motif (WxxDxI) of T. tepidum and Trv. strain 970 (red box) is not present in the A. vinosum α1-polypeptide. An insertion in the A. tepidum and A. vinosum α1-polypeptides is indicated by an arrow. BChl, bacteriochlorophyll; LH1, light-harvesting complex; RC, reaction center.