Figure 56.
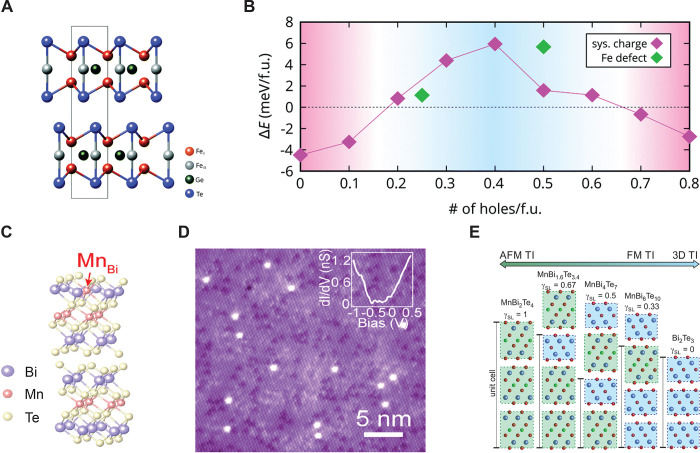
(A, B) The structure and ground states of iron-deficient Fe3–xGeTe2. (A) Side view of stoichiometric Fe3GeTe2. FeI (red) and FeII (silver) are two inequivalent Fe sites with +3 and +2 formal charges, respectively. The FeI–FeI interactions are mostly responsible for interlayer AF ordering while FeI-FeII and FeII–FeII couplings are FM. With Fe defects or doping, FeI–FeII and FeII–FeII become dominant and push interlayer ordering into FM. (B) The calculated energy differences (ΔE) between interlayer AF and FM phases as a function of hole concentration. For ΔE > 0, FM is favored (between 0.2 and 0.6 holes per formula unit). Panels (A) and (B) are adapted with permission from ref (615). Copyright 2020 American Chemical Society. (C) The layered crystal structure of MBT. The red arrow indicates the Mn sites in which antisites have been identified. (D) STM of the surface of a cleaved MBT crystal; white spots show the presence of multiple antisite point defects. Panels (C) and (D) are adapted with permission from ref (616). Copyright 2020 American Chemical Society. (E) Crystal structure representation of the (MnBi2Te4)m(Bi2Te3)n series, ranging from AF to FM with various compositions derived from codepositional MBE. Here, we see that with the addition of Bi2Te3 layers, the MnBi2Te4 layers cannot be coupled together and the material becomes less AF. This is a prime example of an off-stoichiometry defect. Panel (E) adapted with permission from ref (617). Copyright 2020 AIP Publishing.