Abstract
Simulation models are used widely in pharmacology, epidemiology and health economics (HEs). However, there have been no attempts to incorporate models from these disciplines into a single integrated model. Accordingly, we explored this linkage to evaluate the epidemiological and economic impact of oseltamivir dose optimisation in supporting pandemic influenza planning in the USA. An HE decision analytic model was linked to a pharmacokinetic/pharmacodynamics (PK/PD) – dynamic transmission model simulating the impact of pandemic influenza with low virulence and low transmissibility and, high virulence and high transmissibility. The cost-utility analysis was from the payer and societal perspectives, comparing oseltamivir 75 and 150 mg twice daily (BID) to no treatment over a 1-year time horizon. Model parameters were derived from published studies. Outcomes were measured as cost per quality-adjusted life year (QALY) gained. Sensitivity analyses were performed to examine the integrated model's robustness. Under both pandemic scenarios, compared to no treatment, the use of oseltamivir 75 or 150 mg BID led to a significant reduction of influenza episodes and influenza-related deaths, translating to substantial savings of QALYs. Overall drug costs were offset by the reduction of both direct and indirect costs, making these two interventions cost-saving from both perspectives. The results were sensitive to the proportion of inpatient presentation at the emergency visit and patients’ quality of life. Integrating PK/PD–EPI/HE models is achievable. Whilst further refinement of this novel linkage model to more closely mimic the reality is needed, the current study has generated useful insights to support influenza pandemic planning.
Key words: Clinical pharmacology, cost-effectiveness analysis, cost-utility analysis, epidemiology, health economics, oseltamivir
Introduction
Simulation models are widely used in pharmacology, epidemiology and health economics (HEs). These models can explicitly link concepts and ideas to data in order to produce outcomes that are useful to healthcare decision makers [1]. Importantly, there have been no attempts to incorporate the models from pharmacology, epidemiology and HEs into a single integrated model.
While still performed infrequently, the importance of epidemiological modelling to inform economic analyses of prevention and treatment strategies for infectious diseases is well accepted [2]. Cost-effectiveness analyses (CEAs) are typically conducted using static models in which the probability of disease exposure is independent of interventions designed to treat or prevent it. While this is realistic for non-transmissible diseases, it is not suited for communicable diseases given interventions in such circumstance can have a significant impact on disease exposure. For example, vaccines can reduce the susceptible population and antiviral treatments can reduce viral shedding, thus reducing viral transmission. Accordingly, dynamic transmission models are needed when there is interdependence between intervention and disease exposure [3]. These models allow the indirect or herd immunity effect to be predicted. Indeed, static and dynamic models produced dramatically different results [4, 5]. Importantly, a recent review on economic evaluations of interventions for pandemic influenza planning reported most of the studies have used static models [6].
In pharmacology, population pharmacokinetic/pharmacodynamic (PK/PD) models are used to identify optimal dosing regimens [7] and were used recently to demonstrate that elevated doses of oseltamivir may result in reduced viral shedding [8]. This has important implications for the economic evaluation of influenza pandemic planning as influenza viral transmission is a function of viral shedding [9]. These indirect effects, which can be larger than the direct effects [10], are often neglected in cost-effectiveness analyses. Any economic analysis failing to consider these indirect effects will have inaccuracies [11].
Globally, the emergence or re-emergence of influenza outbreaks has been challenging [12–14]. Several economic evaluation studies have been conducted to facilitate the management of influenza outbreaks [15, 16]. A recent systematic review reported that most economic studies used models that were incapable of incorporating variability of all parameters and did not consider the viral transmission process [10]. Most importantly, existing models did not consider the indirect effects which are crucial in a pandemic mitigation strategy.
The current study explored the feasibility of linking pharmacology, epidemiology and HE models within the realm of anti-influenza treatment for pandemic influenza. We previously published an overall framework for the linking without a full description of HE models [17]. To our knowledge, this is the first CEA to directly integrate data from PK/PD and disease transmission models into the economic analysis.
Methods
Linking of pharmacology and epidemiology to HEs models
Previously, a single semi-mechanistic framework assessed quantitatively the impact of oseltamivir pharmacology and treatment approaches on the burden of influenza infection in a hypothetical population of 100 000 individuals across a 1-year flu season [17]. This model connected three discrete quantitative modules viz. a population pharmacokinetic model for oseltamivir; a PK/PD evaluation of oseltamivir carboxylate (OC, active metabolite) on viral shedding; and a susceptible-exposed-infected-recovered (SEIR) epidemiologic model, adapted to incorporate the impact of antiviral therapy.
Briefly, OC PK variability was incorporated using a published population PK model [18] for OC incorporating 390 healthy and infected subjects ranging from 1–78 years across a dose range of 20–1000 mg. The final covariate model from this population PK analysis included allometric scaling and a relationship between weight on the OC central volume of distribution and between weight and creatinine clearance on OC clearance. From this final covariate model, OC PK parameter profiles in 5000 70-kg adult patients aged 18–65 with normal renal function receiving the standard 75 mg twice daily (BID) and 150 mg BID oseltamivir for 5 days was simulated. The area under the concentration-time curve (AUCs) for each patient were quantified and the fraction of patients with OC AUCs above a published PK/PD value associated with faster cessation of viral shedding (14 180 ng.h/ml) [8] was calculated for each dosing regimen; and denoted as FAUChigh.
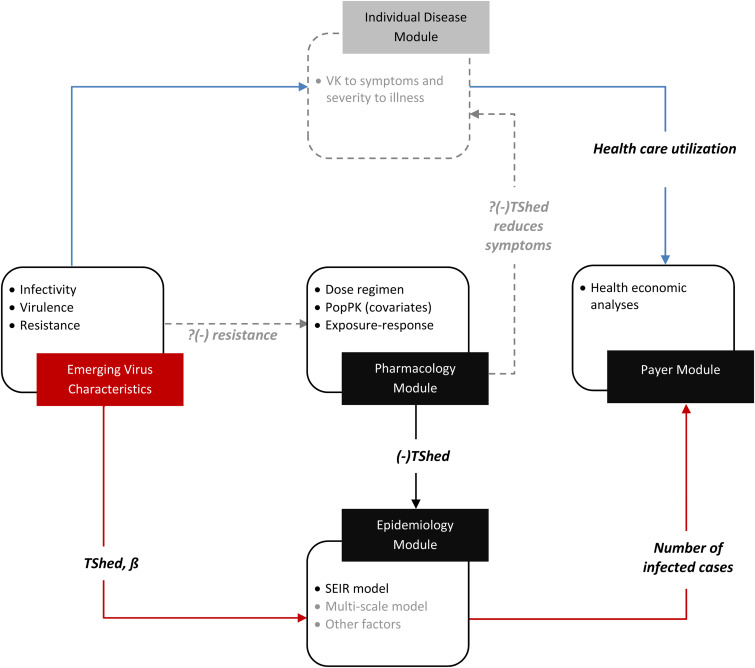
PD variability was integrated via incorporating viral shedding (Tshed) distributions for placebo subjects, and those above and below the PK/PD target as obtained from literature [8]. These PK/PD distributions and other SEIR input parameters (Table 1), allowed the average number of infected cases per 100 000 population to be determined for a range of scenarios relating to dose, viral transmissibility and treatment uptake (vide infra). The important innovation in the current work is the extension of the aforementioned quantitative framework to a decision analytic model to estimate the cost and outcomes of interventions of interest (Fig. 1). Both virus transmissibility and impact of anti-influenza agents alter the number of infected cases entering into the decision analytic model. As previously described, ß governs transmissibility, and is a composite of both frequencies of individual interactions, and the probability that an interaction will result in a successful influenza infection in a susceptible individual [17]. The impact of antiviral treatment is via reducing the time an infected subject is infectious; governed by the duration of viral shedding. As an individual disease model is currently unavailable (Fig. 1), the severity of illness associated with influenza virulence is assumed to directly alter the probabilities of complications and associated costs in the decision analytic model.
Table 1.
Parameters used in the SEIR model
| Descriptor | Unit | Value |
|---|---|---|
| Population size, N | Case | 100 000 |
| Latency period, 1/κ | Day | 1 |
| FAUChigh | ||
| 150 mg BID, P (AUC >14 180 ng.h/ml) | 0.795 ± 0.095 | |
| 75 mg BID, P (AUC > 14 180 ng.h/ml) | 0.326 ± 0.048 | |
| Duration of viral shedding, γ | Day−1 | |
| No treatment, γ0 | 6 ± 2.5 | |
| Oseltamivir 150 mg BID, γhigh (AUC > 14 180 ng.h.ml) | 1.9 *Exp(normal(0,0.51)) | |
| Oseltamivir 75 mg BID, γlow (AUC 0 to 14 180 ng.h/ml) | 3 *Exp(normal(0,0.58)) | |
| Transmission rate, ß | Day−1 | |
| High | 0.41 | |
| Low | 0.21 |
κ, the delay rate between exposure to influenza and symptom development; FAUChigh, the mean (SD) fraction of the simulated population receiving oseltamivir with an AUC > 14 180 ng.h/ml, obtained from the pharmacology module; γ0, the duration of viral shedding under no treatment; γlow, the mean (SD) duration of viral shedding if OC AUC is <14 180 ng.h/ml; γhigh, the mean (SD) duration of viral shedding if OC AUC is >14 180 ng.h/ml, ß, the rate of infectivity; AUC, area under the concentration–time curve; BID, twice daily.
Fig. 1.
Overarching Pharmacology to payer system including ‘modules’. The solid lines indicate that adequate data exists to be able to create semi-mechanistic links to each of adjacent ‘modules’. The dotted lines and light grey describe where significant unknowns remain and are not mature enough to have been incorporated into the current framework. PopPK, population pharmacokinetic; SEIR, susceptible-exposed-infected-recovered; ß, the rate of infectivity; TShed, viral shedding; VK, viral kinetics.
Health economics model structure and data inputs
The decision analytic model (Fig. 2) was developed to evaluate the cost and outcomes of oseltamivir standard (75 mg BID) or high (150 mg BID) dose or no treatment under pandemic influenza scenarios. The number of infected patients entering into the model was simulated from the SEIR model. A cost-utility analysis was undertaken based on the US population of healthy adults, aged 18–64 years old from the payer and societal perspective. Societal perspective included direct and indirect costs. Total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were determined over a 1-year time horizon.
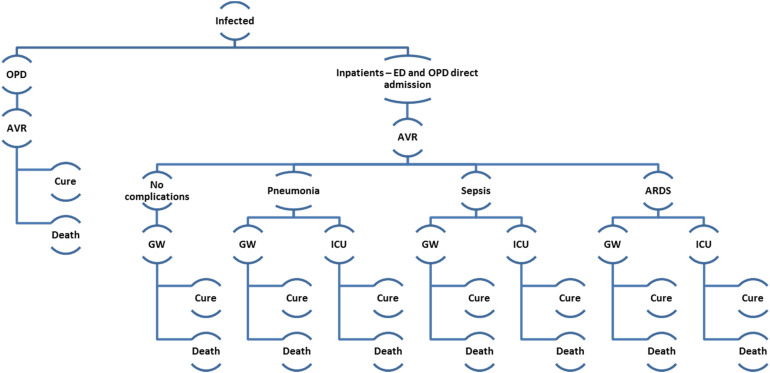
Fig. 2.
Decision analytic model. Influenza patients entered the decision analytic model from epidemiology model. They received treatment in outpatient or inpatient setting. OPD, outpatient; ED, emergency department; AVR, antiviral; GW, general ward; ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
The infected individual entered the HE model either as an outpatient or inpatient (Fig. 2). Those admitted as inpatient would be admitted to a general ward or an intensive care unit, may experience either pneumonia, sepsis and acute respiratory distress syndrome (ARDS) [19–21]. We assumed that patients could only experience one influenza-related complication. The infected patient either recovered from the infection or died. We assumed that oseltamivir was prescribed within 48 h of influenza symptoms and all patients were 100% adherent to treatment received.
A conservative approach involving oseltamivir acting only by reducing the duration of viral shedding and not directly on transmission rate was adopted. As such, only drug treatment effects and not prophylaxis effects were captured in the model. We assumed that oseltamivir reduces the time of symptom alleviation by 21 h and conservatively has no effects on complication, hospitalisation and mortality rate [22–25]. Data about influenza disease progression, medical resource utilisation, cost of treatment and health state utilities were obtained from the literature (Medline searches) and Healthcare Cost and Utilization Project Nationwide Inpatient Sample database [26]. Where possible, data related to the 2009 pandemic H1N1 influenza was used. Meta-analyses were performed when applicable. All costs were converted to 2013 US dollars using the Consumer Price Index [27]. Base-case estimates and ranges for probabilities, costs, utilities and length of stay for hospitalisation are shown in Table 2; detailed descriptions available in Appendix 1.
Table 2.
Input parameters, values and data sources used in the health economics model
| Parameters | Base-case value | 95% CI | SE | Distribution | Source(s) |
|---|---|---|---|---|---|
| Probabilities | |||||
| Medical care received | |||||
| Outpatient visit | 0.972 | NA | [28] | ||
| Inpatient | 0.028 | NA | [28] | ||
| Channels of inpatient admission | |||||
| Through ED visit | 0.778 | NA | [29] | ||
| Through outpatient visit | 0.222 | NA | [29] | ||
| Complication associated with influenza | |||||
| No complications | 0.383 | ||||
| Pneumonia | 0.403 | 0.356–0.450 | Dirichlet | [19–21] | |
| Sepsis | 0.089 | 0.056–0.122 | Dirichlet | [20, 21] | |
| ARDS | 0.125 | 0.086–0.163 | Dirichlet | [20, 21] | |
| Type of hospitalisation | |||||
| No complication | |||||
| ICU | 0 | ||||
| GW | 1 | NA | Assumed | ||
| Pneumonia | |||||
| ICU | 0.518 | 0.045 | Beta | [19] | |
| GW | 0.482 | ||||
| Sepsis | |||||
| ICU | 0.511 | 0.001 | Beta | [30] | |
| GW | 0.489 | ||||
| ARDS | |||||
| ICU | 1 | NA | [21] | ||
| GW | 0 | ||||
| Probable outcome from the medical care received | |||||
| GP | |||||
| Cure | 0.9999 | ||||
| Death | 0.0001 | 0.00007–0.00016 | Beta | [31] | |
| No complication | |||||
| In GW | |||||
| Cure | 0.903 | ||||
| Death | 0.097 | 0.063–0.131 | Beta | [20, 21] | |
| Pneumonia | |||||
| In GW | |||||
| Cure | 0.493 | 0.399–0.586 | Beta | [20, 21] | |
| Death | 0.507 | ||||
| In ICU | |||||
| Cure | 0.489 | ||||
| Death | 0.511 | 0.418–0.604 | Beta | [20, 21] | |
| Sepsis | |||||
| In GW | |||||
| Cure | 0.081 | 0–0.185 | Beta | [20, 21] | |
| Death | 0.919 | ||||
| In ICU | |||||
| Cure | 0.257 | ||||
| Death | 0.743 | 0.575–0.911 | Beta | [20, 21] | |
| ARDS | |||||
| In GW | |||||
| Cure | 0.002 | 0–0.016 | Beta | [20, 21] | |
| Death | 0.998 | ||||
| In ICU | |||||
| Cure | 0.151 | ||||
| Death | 0.849 | 0.520–1 | Beta | [20, 21] | |
| Costs (USD, year of costing: 2013) | |||||
| Direct medical care costs | |||||
| Oseltamivir | 132.77a | NA | [32] | ||
| Over the counter medications | 16.95 | 12.70–21.20 | Log normal | [21] | |
| GP visit | 169b | NA | [28] | ||
| ED visit | 551 | 446–656 | Log normal | [33] | |
| Hospitalisation | |||||
| GW | |||||
| No complication | 17 260c | 1592 | Log normal | [26] | |
| Pneumonia | 18 966c | 1500 | Log normal | [26] | |
| Sepsis | 23 771c | 466 | Log normal | [26] | |
| ARDS | 45 330 | 0–93 348 | Log normal | [34] | |
| ICU | |||||
| Pneumonia | 22 771 | 36 890d | Log normal | [35] | |
| Sepsis | 44 958 | 69 698d | Log normal | [36] | |
| ARDS | 128 860 | 85 738–170 461 | Log normal | [34] | |
| Direct non-medical care cost | |||||
| Transportation (per visit) | 2.83e | NA | [37, 38] | ||
| Indirect costs (daily productivity loss by age) | |||||
| Age 18–64 | 146.04f | NA | [39] | ||
| Productivity loss (days lost) = length of stay plus days of convalescenceg | |||||
| GP visit | 2.0b | [28,40] | |||
| Hospitalisation | |||||
| GW | |||||
| No complication | 7.4c | [26, 40] | |||
| Pneumonia | 8.4c | [26, 40] | |||
| Sepsis | 10.5c | [26, 40] | |||
| ARDS | 13.0 | [34,40] | |||
| ICU | |||||
| Pneumonia | 9.7 | [35,40] | |||
| Sepsis | 14.4 | [36,40] | |||
| ARDS | 17.0 | [34,40] | |||
| Length of stay (days) | |||||
| GP visit | 1.0 | NA | [28] | ||
| Hospitalisation | |||||
| GW | |||||
| No complication | 6.4 | 0.4 | [26] | ||
| Pneumonia | 7.4 | 0.5 | [26] | ||
| Sepsis | 9.5 | 0.2 | [26] | ||
| ARDS | 12.0 | 15.0d | [34] | ||
| ICU | |||||
| Pneumonia | 8.7 | 9.9d | [35] | ||
| Sepsis | 13.4 | 16.0d | [36] | ||
| ARDS | 16.0 | 22.0d | [34] | ||
| Utilities | |||||
| Baseline average quality of life | 0.96 | 0.92–1.00 | Beta | [41, 42] | |
| Quality of life during illness with influenza | 0.81 | 0.70–0.90 | Beta | [41, 43] | |
| Pneumonia | 0.63 | 0.06d | Beta | [44] | |
| Sepsis in hospital ward | 0.59 | 0.02d | Beta | [45–48] | |
| Sepsis in ICU | 0.10 | 0.08–0.15 | Beta | [43–45] | |
| ARDS in hospital ward | 0.59 | 0.02d | Beta | [45, 49] | |
| ARDS intubated in ICU | 0.10 | 0.08–0.15 | Beta | [45, 49] | |
| Recovery from severe influenza, for patients who received inpatient ICU care | 0.90h | 0.85–0.95 | Beta | [41] |
CI, confidence interval; SE, standard error; ED, emergency department; GP, general practitioner; GW, general ward; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; USD, United States Dollar; AWP, average wholesale price.
Oseltamivir is available as 75 mg/capsule and in a pack of 10 capsules in the USA. The AWP for one pack of oseltamivir is USD 132.77.
Weighted probability or cost estimate was calculated using probability or cost reported for two age groups, i.e. 18–49 and 50–64 years, assuming the age-distribution of the influenza adults is similar to that of the U.S. population in 2012 [50].
Weighted cost or length of stay estimate was calculated using cost or length of stay reported for two age groups, i.e. 18–44 and 45–64 years.
Reported as standard deviation.
Calculated by multiplying 5 miles round trip and the standard business mileage rate 2013 for the use of a car.
Calculated using the age- and sex-stratified median weekly earnings in the U.S. Bureau of Labor Statistics for the age group 18–64 years, taking account of the unemployment rate of 7.3%.
Calculated using the length of stay reported and adding one additional day for convalescence [40].
Khazeni et al. [41] adjusted for possible on-going disability following recovery from severe (all patients who received inpatient ICU care) influenza, which assumed an estimated quality of life of 0.9 for the remainder of those patients’ lifetimes. In our HE model, we assumed the value of 0.90 for the rest of the year for those patients.
Simulation scenarios and treatment comparison
We investigated the HE impact of two extreme hypothetical pandemic scenarios, namely, influenza virus with low virulence and low transmissibility and virus with high virulence and high transmissibility [51, 52]. The ß values for transmissibility were from Kamal et al. [17], (see Table 1) whereas virulence was based on health care utilisation; low virulence was based on pH1N1 experience [12]; and the high virulence scenario involved doubling the probability of hospitalisation [53] for the low virulence scenario within the decision analytic model.
We evaluated three different management strategies: (1) no treatment, (2) the standard approved oseltamivir dose (75 mg BID) and (3) high dose (150 mg BID). We also evaluated the treatment strategies according to varying treatment uptake rates (25, 50 and 80%), defined as the percentage of infected subjects treated, across an infected population. Detailed descriptions of all values are available in Appendix 1. Analyses were performed using Berkeley Madonna™ version 8.3.18, R version 2.15.3 and Microsoft Excel 2010 (Redmond, Washington).
Sensitivity analyses
One-way sensitivity analyses were performed to investigate the effects of altering parameters within the plausible ranges (Table 2). Additional sensitivity analysis was undertaken to determine the robustness of estimates when a different approach for productivity loss estimation was adopted. In the base-case analyses, we adopted the approach in Meltzer et al. [40] Alternatively, we calculated productivity loss using the relationship between ‘time to return to normal activity’ and ‘illness duration’ (Appendix 1). We also varied the probability of developing ARDS in highly virulent and highly transmissible pandemic influenza case. Probabilistic sensitivity analysis (PSA) was undertaken to address uncertainty in the assumptions underlying the model by allowing each input parameter values to vary simultaneously over their respective feasible ranges within the model; 5000 second-order Monte Carlo simulations were performed in which the parameter values were drawn from pre-defined distributions. Results from the PSA are presented as scatterplots in cost-effectiveness plane.
Results
Low virulence and low transmissibility
Based on a hypothetical cohort of 100 000 individuals, under low virulence and low transmissibility, standard-dose oseltamivir (75 mg BID) was cost-saving from both perspectives compared to no treatment (Table 3). It could save USD30,246,490 (USD81,272,885), USD33,352,767 (USD93,993,932) and USD30,406,894 (USD92,614,326) for oseltamivir uptakes of 25%, 50% and 80% by averting 706, 412 and 783 deaths, respectively, from payer (societal) perspective. This translates to 361, 430 and 441 QALYs gained for uptakes of 25%, 50% and 80%, respectively.
Table 3.
Base-case analyses: high-dose vs. no treatment, and standard dose vs. no treatment
| Payer perspective | Societal perspective | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparators | Δ Costsa (payer) | Δ Costsa (societal) | Δ Death | Δ LYs | Δ QALYs | Cost per LY gained | Cost per QALY gained | Cost per LY gained | Cost per QALY gained |
| Low virulence and low transmissibility | |||||||||
| 75 mg vs. no treatment | |||||||||
| 25% uptake | −30 246 490 | −81 272 885 | −706 | 335 | 361 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 50% uptake | −33 352 767 | −93 996 756 | −412 | 399 | 430 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 80% uptake | −30 406 894 | −92 614 326 | −783 | 410 | 441 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 150 mg vs. no treatment | |||||||||
| 25% uptake | −29 839 058 | −85 192 106 | −376 | 364 | 392 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 50% uptake | −27 742 305 | −89 785 170 | −423 | 409 | 441 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 80% uptake | −20 483 671 | −83 687 273 | −430 | 416 | 449 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 150 vs. 75 mg | |||||||||
| 25% uptake | 407 432 | −3 919 221 | −30 | 29 | 31 | 14 018 | 13 117 | Cost-saving | Cost-saving |
| 50% uptake | 5 610 462 | 4 110 702 | −11 | 10 | 11 | 546 753 | 515 260 | 400 598 | 377 524 |
| 80% uptake | 9 923 223 | 8 927 052 | −7 | 7 | 7 | 1 423 516 | 1 353 188 | 1 280 612 | 1 217 344 |
| High virulence and high transmissibility | |||||||||
| 75 mg vs. no treatment | |||||||||
| 25% uptake | −11 885 337 | −27 413 072 | −168 | 162 | 174 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 50% uptake | −49 309 677 | −101 904 191 | −617 | 598 | 629 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 80% uptake | −88 899 525 | −179 850 525 | −1098 | 1063 | 1112 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 150 mg vs. no treatment | |||||||||
| 25% uptake | −24 304 917 | −53 631 551 | −341 | 331 | 349 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 50% uptake | −63 252 396 | −133 577 886 | −844 | 818 | 856 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 80% uptake | −95 501 955 | −200 976 888 | −1288 | 1247 | 1302 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 150 vs. 75 mg | |||||||||
| 25% uptake | −12 419 581 | −26 218 479 | −174 | 168 | 174 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 50% uptake | −13 942 719 | −31 673 695 | −227 | 220 | 227 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
| 80% uptake | −6 602 430 | −21 126 363 | −190 | 184 | 189 | Cost-saving | Cost-saving | Cost-saving | Cost-saving |
All costs are expressed in 2013 USD.
High-dose oseltamivir (150 mg BID) when compared to no treatment was also cost-saving from payer (societal) perspective (Table 3), leading to cost-saving of USD29,839,058 (USD85,192,106), USD27,742,305 (USD89,785,170) and USD20,483,671 (USD83,687,273) for uptakes of 25%, 50% and 80% by preventing 376, 423 and 430 deaths, respectively, leading to the associated QALY gains of 392, 441 and 449, respectively.
Compared to low-dose, high-dose oseltamivir would avert −30, −11, −7 deaths at 25%, 50% and 80% uptakes, respectively, leading to the associated QALY gains of 31, 11, 7, respectively. It led to overall increased cost of USD407,432, USD5,610,462, USD9,923,223 from payer perspective for uptakes of 25%, 50%, 80% uptakes, respectively, resulting in USD13,117, USD546,753, USD1,353,188 per QALY gained. From societal perspective, using high-dose oseltamivir could save USD3,919,221 at 25% uptake (cost-saving) but incurred extra cost of USD4,110,702 and USD8,927,052 at 50% and 80% uptakes, leading to USD377,524 and USD1,217,344 QALY gained, respectively.
High virulence and high transmissibility
Under the scenario of high virulence and high transmissibility, compared with no treatment, oseltamivir 75 mg BID was dominant (Table 3). It could avert 168, 617 and 1098 deaths, translating to 174, 598 and 1063 QALYs gained for the uptakes of 25%, 50% and 80%, respectively (Table 3). The standard-dose could save USD11,885,337 (USD27,413,072), USD49,309,677 (USD101,904,191) and USD88,899,525 (USD179,850,525) for 25%, 50% and 80% uptakes from payer (societal) perspective.
When high-dose oseltamivir was compared to no treatment (Table 3), it was shown to save USD24,304,917 (USD53,631,551), USD63,252,396 (USD133,577,886) and USD95,501,955 (USD200,976,888) for uptakes of 25%, 50% and 80%, by preventing 341, 844 and 1288 deaths, respectively, leading to the associated QALY gains of 330, 818 and 1247, respectively.
Compared to low-dose, our model indicated that high-dose oseltamivir would save USD12,419,581 (USD26,218,479), USD13,942,719 (USD31,673,695) and USD6,602,430 (USD21,126,363) from the payer (societal) perspective for uptakes of 25%, 50% and 80% (Table 3). In addition, 174, 227 and 190 deaths could be prevented, leading to QALY gains of 174, 227 and 189, respectively.
Sensitivity analyses
The tornado diagrams (Fig. 3) summarise the results of 1-way sensitivity analyses. Under low virulence and low transmissibility, at 80% oseltamivir uptake, the ICER was most sensitive to the probability of hospitalisation for ED cases and baseline average quality of life. Under the high virulence and high transmissibility scenario, the baseline average quality of life and median weekly earnings in the USA were the two most influential factors. The results were also very similar to the other uptakes and thus not shown.
Fig. 3.
Tornado diagram (150 mg vs. no treatment with 80% uptake of oseltamivir): One-way sensitivity analysis under (1a) low virulence and low transmissibility and (1b) high virulence and high transmissibility. (a) Low virulence and low transmissibility. #+/−: The higher value of the parameter leads to lower ICER. $−/+: The higher value of the parameter leads to higher ICER. (b) High virulence and high transmissibility. #+/−: The higher value of the parameter leads to lower ICER.
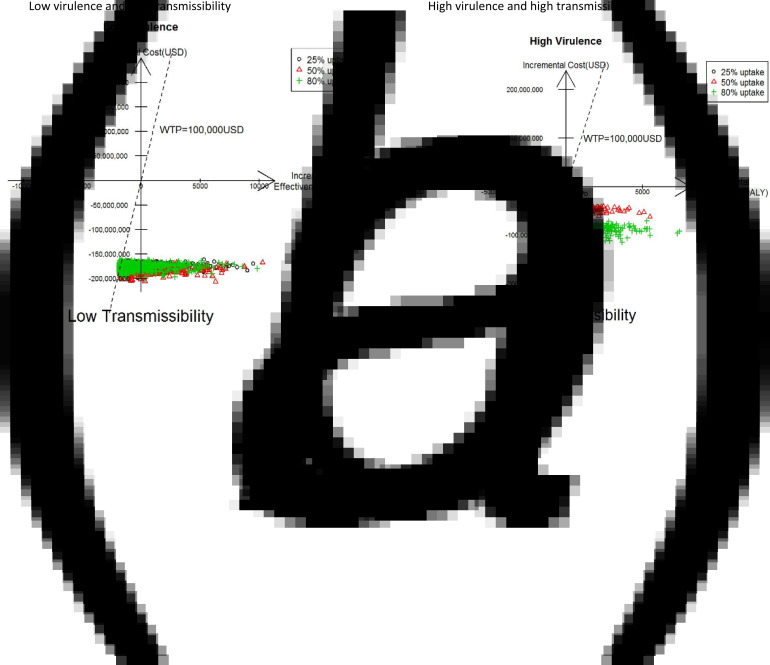
Monte Carlo simulations (5000) for different levels of viral virulence, transmissibility and oseltamivir uptake from the societal perspective are presented in Fig. 4. In both scenarios, a very large proportion of the simulated results fell in the south-east quadrant, suggesting that 75 mg BID vs. no treatment at 25%, 50% and 80% uptakes were highly likely to be cost-saving. The results of 150 mg BID from a societal perspective were also very similar (data not provided).
Fig. 4.
Scatter plots (incremental cost vs. incremental QALY) of 75 mg vs. no treatment under societal perspective. (a) Low virulence and low transmissibility. (b) High virulence and high transmissibility.
Discussion
This is the first study to link pharmacology, epidemiology and HE models into a single integrated model. We examined the costs and outcomes of different levels of oseltamivir dosing for pandemic influenza planning using the information from the PK/PD model to ultimately inform an HE model by way of an SEIR model. While we applied this novel linked model within the context of pandemic influenza, its usefulness is not limited to this arena. Our approach can also be useful for policy-oriented decision making surrounding the use of other antimicrobials agents.
Indeed, our results show that using standard- or high-dose oseltamivir will afford a significant reduction in deaths due to three major influenza-related complications over a 1-year time horizon, thus leading to substantial savings in both life years and QALYs under various degrees of virus virulence and transmissibility. The extra cost for oseltamivir was offset by reductions in the number of disease episodes and influenza-related deaths, resulting in substantial savings.
Several economic evaluations on influenza pandemic preparedness strategies have been published, including studies evaluating vaccine, pharmaceutical and non-pharmaceutical (e.g. social distancing) interventions with the majority focusing on anti-influenza drugs [10]. Pharmaceutical interventions combined with non-pharmaceutical interventions have been found to be relatively cost-effective in comparison to vaccines and/or antivirals alone. However, pharmaceutical interventions vary from cost-saving to being not cost-effective [10]. The wide range of cost-effectiveness results is due to several factors such as different dosages and durations for prophylaxis as well as the comparator. Our results showing cost-savings of oseltamivir under all scenarios and dosages were possibly due to the absence of consideration of concomitant treatments and the social contact structure among various subpopulations.
Despite the implementation of the World Health Organization's (WHO's) pandemic preparedness plan and response guide during the 2009 pandemic H1N1, experience and evidence suggest that the global community is ill-prepared to respond to a severe influenza pandemic or to any similarly global, life-threatening public-health emergency [54]. The possibility of the avian H5N1 becoming pandemic has raised concern and has been anticipated by the public health authorities [55]. This strain is a significant threat given its high fatality rate in humans, the large reservoir of poultry in South-East Asia and that H5N1 influenza may be genetically modified to become more transmissible [51, 56, 57]. Importantly, the global community should be prepared for the next pandemic by having a full understanding of the consequences of influenza pandemics and their related interventions. In the current study, by using the 2009 pandemic data in the linked models, we explored the cost-effectiveness of oseltamivir as a pandemic mitigation strategy in two hypothetical pandemic scenarios with different severity, inspired by the 2009 pandemic H1N1 and H5N1. Our study did not consider the reported case fatality ratio (CFR) of H5N1 by the WHO due to inconclusive findings on the real CFR estimate by different sources [58, 59]. Furthermore, the CFR may decrease as more information becomes available or as the epidemic progresses. Several recent studies from the Cochrane collaboration [60] and Muthuri et al. [61] have called into question the efficacy of oseltamivir. Therefore, we conservatively estimated the drug effect of oseltamivir based solely on viral shedding in our pharmacology component of the integrated model. Subsequently, in the HEs model, assumptions were made that oseltamivir shortened the duration of influenza symptoms by 21–22 h, but had no effect on the progression to secondary complications, hospitalisation or mortality [22–24]. These assumptions may underestimate the true effects of oseltamivir during an influenza pandemic.
Our work has several limitations. First, we did not take into consideration other interventions such as the use of masks, school closure and influenza vaccine. The concurrent use of other interventions may reduce the impact of oseltamivir. Second, we assumed that all infected cases seek medical care, which might not be true in reality. Third, our model was confined to only a healthy population between 18 and 65 years of age. However, the model could easily be extended to include other patient populations. Finally, it is noteworthy that patients’ viral shedding duration for 75 or 150 mg BID was based upon detailed shedding data from a human inoculation study, where adults receive oseltamivir at 28-h post-inoculation. In reality, the impact on viral shedding will decrease, as patients initiate treatment later in their disease course. Therefore, even though many assumptions above may underestimate the true effects of oseltamivir during an influenza pandemic, delayed initiation of treatment would result in smaller clinical and HE benefits.
Despite these limitations, our study has many, important strengths. First, because we used a dynamic transmission model, we were able to incorporate important indirect effects of oseltamivir treatment. Second, we undertook substantial effort through comprehensive literature review and meta-analysis to synthesise the best available evidence in generating input parameters. We, therefore, feel that our model inputs are of highest quality. Third, not only is our integration of pharmacology into an epidemiological model of influenza transmission novel, it also allows for a more realistic drug effect variability than previous efforts which have relied simply on blunt assumptions as to the efficacy of antivirals in reducing the infectiveness of the virus [62]. Because of the increased realism of the drug effect, the transmission dynamics in our model more closely mirror those that would occur in the real-world. Therefore, the results from our economic analysis will be more reflective of real-life, thus, improving the usability of this economic model for decision makers. Finally, including pharmacology, epidemiology and HEs into one integrated model may facilitate earlier meaningful dialogue between the key stakeholders, such as sponsors, regulators and payers. This concept provides a crucial element for so-called adaptive licensing approaches of drug development such as staggered approval, managed entry and progressive authorisation [63].
Conclusions
This is the first study demonstrating the feasibility of linking of PK/PD–EPI/HE models. Our linked model, which explored the use of oseltamivir in pandemic influenza as an example, allows for direct integration of antiviral effectiveness on viral shedding to the disease transmission model. This, in turn, leads to better understanding of the effects of the different doses of oseltamivir on mortality, morbidity and economic outcomes that make up the outputs of our economic model. Simulations that better reflect reality lead to more informed policy making, which, in turn, can save healthcare costs as well as patient lives. However, efforts are needed to further refine the model to better represent the reality. It enables us to better inform multiple stakeholders even at the early phase of drug development.
Acknowledgement
This work was supported by the funding from F. Hoffmann-La Roche Ltd.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268818000158.
click here to view supplementary material
References
- 1.Weinstein MC, et al. (2003) Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices – modeling studies. Value in Health 6(1), 9–17. [DOI] [PubMed] [Google Scholar]
- 2.Jit M and Brisson M (2011) Modelling the epidemiology of infectious diseases for decision analysis: a primer. PharmacoEconomics 29(5), 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lugner AK, Mylius SD and Wallinga J (2010) Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Economics 19(5), 518–531. [DOI] [PubMed] [Google Scholar]
- 4.Brisson M and Edmunds WJ (2003) Economic evaluation of vaccination programs: the impact of herd-immunity. Medical Decision Making 23(1), 76–82. [DOI] [PubMed] [Google Scholar]
- 5.Brisson M and Edmunds WJ (2006) Impact of model, methodological, and parameter uncertainty in the economic analysis of vaccination programs. Medical Decision Making 26(5), 434–446. [DOI] [PubMed] [Google Scholar]
- 6.Lugner AK and Postma MJ (2009) Mitigation of pandemic influenza: review of cost-effectiveness studies. Expert Review of Pharmacoeconomics and Outcomes Research 9(6), 547–558. [DOI] [PubMed] [Google Scholar]
- 7.Duffull SB, Wright DF and Winter HR (2011) Interpreting population pharmacokinetic-pharmacodynamic analyses – a clinical viewpoint. British Journal of Clinical Pharmacology 71(6), 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner CR, et al. (2013) Pharmacokinetic-pharmacodynamic determinants of oseltamivir efficacy using data from phase 2 inoculation studies. Antimicrobial Agents and Chemotherapy 57(8), 3478–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulik C, et al. Pharmacokinetic-pharmacodynamic (PK-PD) evaluation of the impact of oseltamivir on influenza viral endpoints. 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Washington DC, US.
- 10.Pradas-Velasco R, Antonanzas-Villar F and Martinez-Zarate MP (2008) Dynamic modelling of infectious diseases: an application to the economic evaluation of influenza vaccination. PharmacoEconomics 26(1), 45–56. [DOI] [PubMed] [Google Scholar]
- 11.Pitman R, et al. (2012) Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force working group-5. Medical Decision Making 32(5), 712–721. [DOI] [PubMed] [Google Scholar]
- 12.Girard MP, et al. (2010) The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 28(31), 4895–4902. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation (WHO) (2013) Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2013. Geneva: WHO. [Google Scholar]
- 14.World Health Organization. Global alert and response (GAR). Avian influenza. Geneva: WHO. [Google Scholar]
- 15.Kelso JK, et al. (2013) Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories. BMC Public Health 13, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milne GJ, Halder N and Kelso JK (2013) The cost effectiveness of pandemic influenza interventions: a pandemic severity based analysis. PLoS ONE 8(4), e61504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal MA, et al. (2017) Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. British Journal of Clinical Pharmacology 83(7), 1580–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamal MA, et al. (2013) Population pharmacokinetics of oseltamivir: pediatrics through geriatrics. Antimicrobial Agent and Chemotherapy 57(8), 3470–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain S, et al. (2012) Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus – United States, 2009. Clinical Infectious Diseases 54(9), 1221–1229. [DOI] [PubMed] [Google Scholar]
- 20.Jain S, et al. (2009) Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. New England Journal of Medicine 361(20), 1935–1944. [DOI] [PubMed] [Google Scholar]
- 21.Skarbinski J, et al. (2011) Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States – September–October 2009. Clinical Infectious Diseases 52(suppl. 1), S50–S59. [DOI] [PubMed] [Google Scholar]
- 22.Burch J, et al. (2009) Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technology Assessment 13(58), 1–265, iii–iv. [DOI] [PubMed] [Google Scholar]
- 23.Jefferson T, et al. (2012) Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews 1, CD008965. [DOI] [PubMed] [Google Scholar]
- 24.Hsu J, et al. (2012) Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Annals Internal Medicine 156(7), 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michiels B, et al. (2013) The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS ONE 8(4), e60348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HCUP Nationwide Inpatient Sample (NIS) (2009) Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- 27.U.S. Bureau of Labor Statistics (2013) CPI Inflation Calculator. Washington DC, US: U.S. Bureau of Labor Statistics. [Google Scholar]
- 28.Molinari NA, et al. (2007) The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25(27), 5086–5096. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez MK, et al. (2013) The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corporation. [Google Scholar]
- 30.Angus DC, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 29(7), 1303–1310. [DOI] [PubMed] [Google Scholar]
- 31.Presanis AM, et al. (2009) The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Medicine 6(12), e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Physician Desk Reference (2013) PDR Red Book: Pharmacy's Fundamental Reference. Montvale: Thompson Healthcare Inc. [Google Scholar]
- 33.Caldwell N, et al. (2013) “How much will I get charged for this?” patient charges for top ten diagnoses in the emergency department. PLoS ONE 8(2), e55491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesen J, et al. (2012) Relative cost and outcomes in the intensive care unit of acute lung injury (ALI) due to pandemic influenza compared with other etiologies: a single-center study. Annals Intensive Care 2(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, et al. (2012) Clinical and economic burden of community-acquired pneumonia in the medicare fee-for-service population. Journal of the American Geriatrics Society 60(11), 2137–2143. [DOI] [PubMed] [Google Scholar]
- 36.MacLaren R, et al. (2008) Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Critical Care Medicine 36(12), 3184–3189. [DOI] [PubMed] [Google Scholar]
- 37.Internal Revenue Service (2013) Standard Mileage Rates for 2013. Washington DC, US: IRS. [Google Scholar]
- 38.Center of Disease Prevention (2009) Distance to Nearest Hospital Files, NAMCS and NHAMCS. Atlanta: CDC. [Google Scholar]
- 39.U.S. Bureau of Labor Statistics (2013) Median Weekly Earnings by Age, Sex, Race and Hispanic or Latino Ethnicity, First Quarter 2013. Washington DC, US: U.S. Bureau of Labor Statistics. [Google Scholar]
- 40.Meltzer MI, Cox NJ and Fukuda K (1999) The economic impact of pandemic influenza in the United States: priorities for intervention. Emerging Infectious Diseases 5(5), 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khazeni N, et al. (2009) Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic. Annals Internal Medicine 151(12), 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryback DG, et al. (1993) The beaver dam health outcomes study: initial catalog of health-state quality factors. Medical Decision Making 13(2), 89–102. [DOI] [PubMed] [Google Scholar]
- 43.Turner DA, et al. (2006) The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine 24(7), 1035–1043. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, et al. (2012) The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine 30(24), 3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angus DC, et al. (2001) Quality-adjusted survival in the first year after the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 163(6), 1389–1394. [DOI] [PubMed] [Google Scholar]
- 46.Talmor D, et al. (2008) The costs and cost-effectiveness of an integrated sepsis treatment protocol. Critical Care Medicine 36(4), 1168–1174. [DOI] [PubMed] [Google Scholar]
- 47.Davies A, et al. (2005) Cost effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Anaesthesia 60(2), 155–162. [DOI] [PubMed] [Google Scholar]
- 48.Drabinski AWG and Formica C (2001) Observational evaluation of health state utilities among a cohort of sepsis patients. Value in Health 4(2), 128–129. [Google Scholar]
- 49.Macario A, Chow JL and Dexter F (2006) A Markov computer simulation model of the economics of neuromuscular blockade in patients with acute respiratory distress syndrome. BMC Medical Informatics and Decision Making 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.S. Bureau of Labor Statistics (2012) Household Data, Annual Averages. Washington DC, US: U.S. Bureau of Labor Statistics. [Google Scholar]
- 51.Russell CA, et al. (2012) The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 336(6088), 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawaoka Y (2012) H5n1: flu transmission work is urgent. Nature 482(7384), 155. [DOI] [PubMed] [Google Scholar]
- 53.Lee BY, et al. (2010) To test or to treat? An analysis of influenza testing and antiviral treatment strategies using economic computer modeling. PLoS ONE 5(6), e11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organisation (WHO) (2011) Report of the Review Committee on the Functioning of the International Health Regulations (2005) in Relation to Pandemic (H1N1) 2009. Geneva: WHO. [Google Scholar]
- 55.Fineberg HV (2014) Pandemic preparedness and response – lessons from the H1N1 influenza of 2009. New England Journal of Medicine 370(14), 1335–1342. [DOI] [PubMed] [Google Scholar]
- 56.Herfst S, et al. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336(6088), 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai M, et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486(7403), 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li FC, et al. (2008) Finding the real case-fatality rate of H5N1 avian influenza. Journal of Epidemiology and Community Health 62(6), 555–559. [DOI] [PubMed] [Google Scholar]
- 59.Cowling BJ, et al. (2013) Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 382(9887), 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jefferson T, et al. (2014) Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. British Medical Journal 348, g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muthuri SG, et al. (2014) Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respiratory Medicine 2(5), 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson NM, et al. (2006) Strategies for mitigating an influenza pandemic. Nature 442(7101), 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eichler HG, et al. (2012) Adaptive licensing: taking the next step in the evolution of drug approval. Clinical Pharmacology and Therapeutics 91(3), 426–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268818000158.
click here to view supplementary material