Fig. 2. bGSDMs are associated with proteases, defend from phages, and execute cell death.
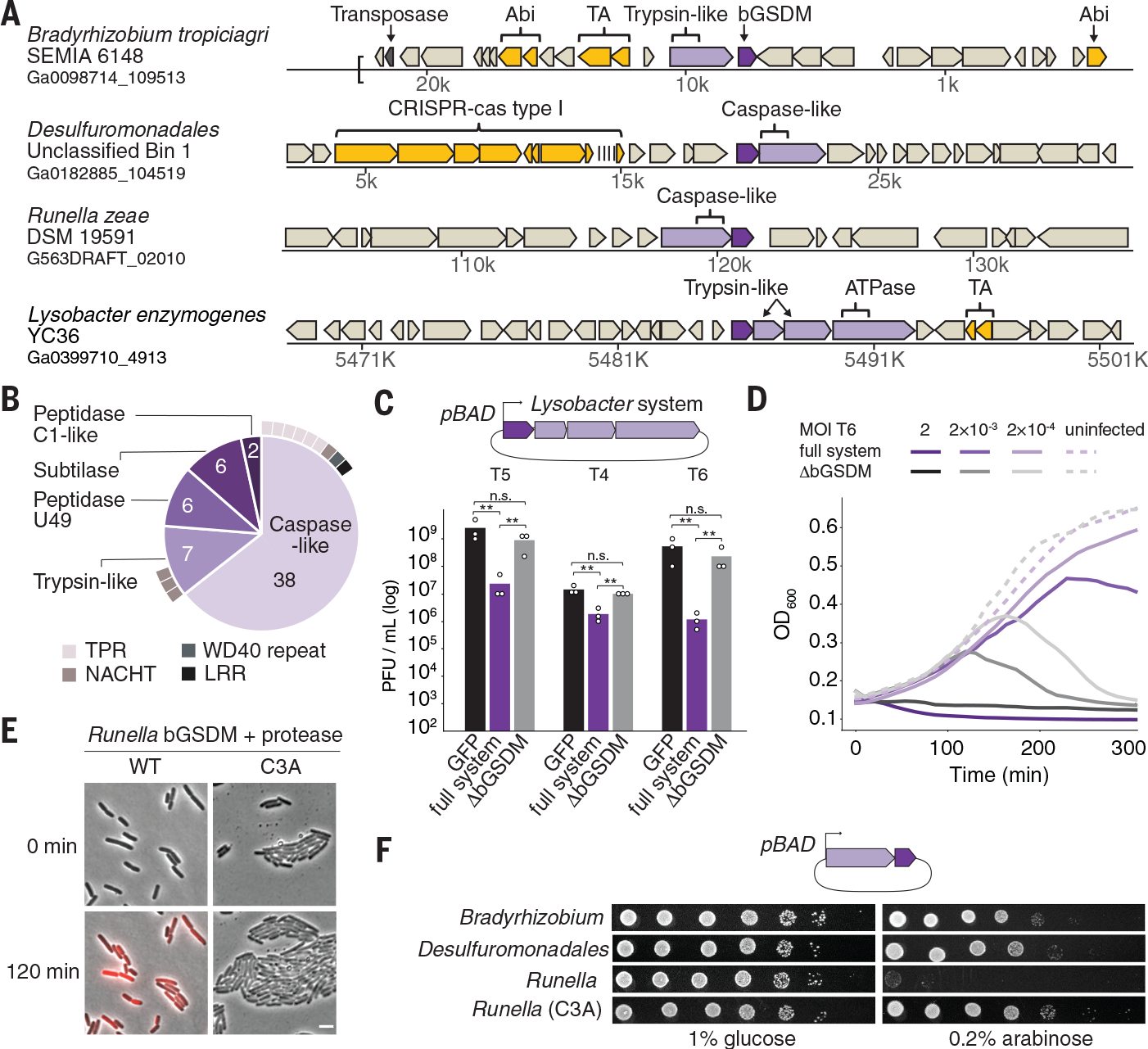
(A) Representative instances of bGSDMs and associated proteases, in their genomic neighborhoods. Genes known to be involved in anti-phage defense are shown in yellow. TA, toxin-antitoxin; Abi, abortive infection; ATPase, adenosine triphosphatase. (B) Types of proteases found adjacent to bGSDMs (n = 59). Some bGSDMs appear with more than one adjacent protease. Caspase-like proteases include peptidase C14 (n = 15) and CHAT (n = 23). Cases in which the protease gene also encodes an additional domain are indicated. TPR, tetratricopeptide repeat; LRR, leucine-rich repeat. (C) A bGSDM-containing operon protects against phages. The efficiency of plating of phages on E. coli MG1655 cells expressing the Lysobacter bGSDM WT or mutated operon is shown. Data represent plaque-forming units (PFU) per milliliter and are the averages of three independent replicates, with individual data points overlaid. GFP represents a control strain. Statistical significance was determined by a one-way analysis of variance (ANOVA) and Tukey multiple comparison test. Not significant (n.s.) ≥ 0.05, **P = 0.001 to 0.01. (D) Growth of liquid cultures of E. coli expressing the WT and mutated Lysobacter bGSDM operons. Cells were infected with phage T6. For each experiment, data represent one out of three biological replicates (replicates are shown in Fig. S6). OD600, optical density at 600 nm. (E) The Runella bGSDM operon causes cell death. E. coli DH5α cells expressing the Runella protease and WT or C3A mutated bGSDM were examined by time lapse microscopy. Overlay images from PI (red) and phase contrast of cells captured at the start of the experiment and after 120 min of incubation are shown. Scale bar, 2 μm. (F) bGSDM operons are toxic. Cells encoding protease and WT or mutated bGDSM were plated in 10-fold serial dilution on LB-agar in conditions that repress operon expression (1% glucose) or induce expression (0.2% arabinose).
