Figure 1. Desialylation of the recombinant AIM-A1 fragment did not change its monomeric state.
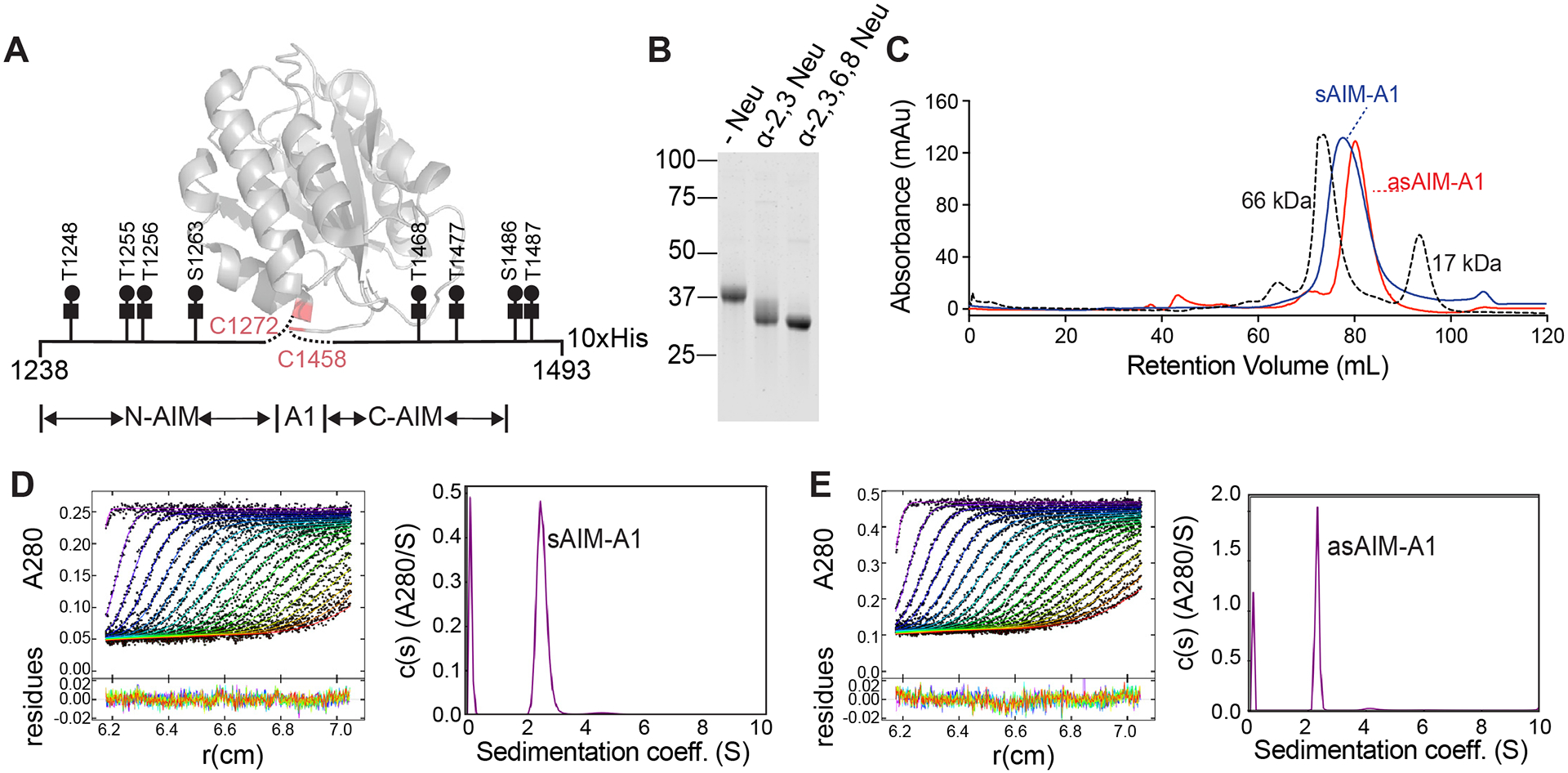
(A) Schematic of recombinant AIM-A1 with Core-1 O-glycosylation sites listed. The ribbon diagram of A1 domain is drawn using PDB 1sq0. (B) Recombinant AIM-A1 was incubated with (+) or without (−) neuraminidases as indicated for 15 min at 37°C. Desialylation was confirmed via SDS-PAGE stained with GelCode Blue. (C) Gel-filtration chromatography using Superdex 200 16/600 pg column of sAIM-A1 and asAIM-A1 with BSA (66 kDa) and myoglobin (17 kDa) shown as standards. Samples were loaded at 1 mg/mL with a flowrate of 1 mL/min. (D, E) Sedimentation velocity results of sAIM-A1 and asAIM-A1 showing the fitted absorbance scans and residuals (left) and sedimentation coefficient distributions (right).
