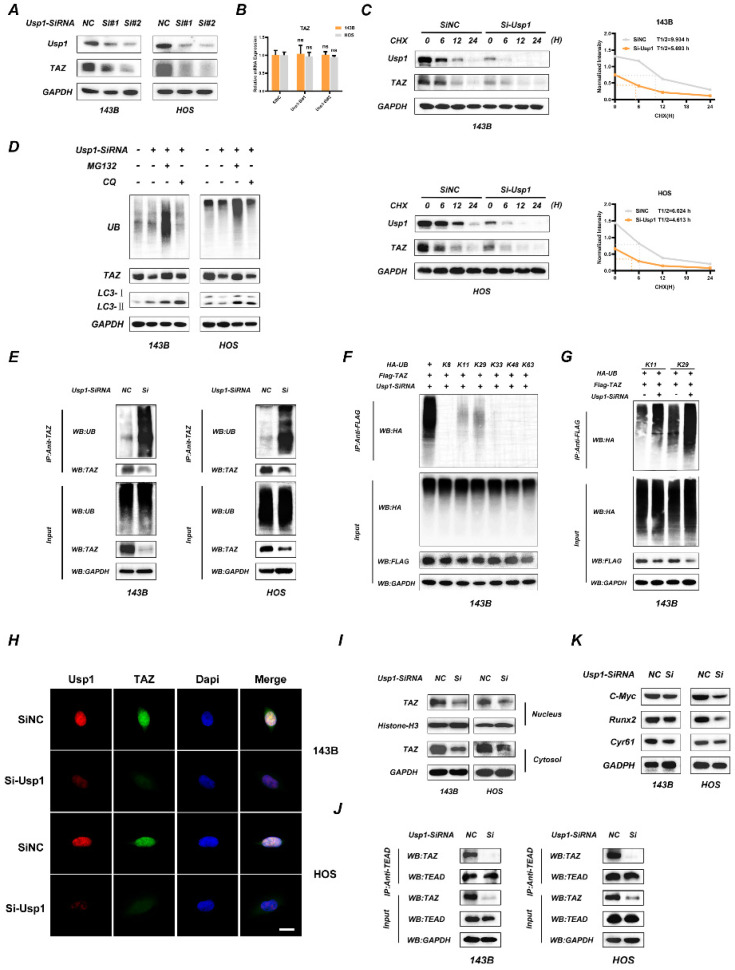
Figure 2.
USP1 depletion promotes the ubiquitination of TAZ and results in dysfunctional Hippo signaling pathway. A, B. USP1 depletion significantly decreased TAZ protein levels in OS cells, as determined by Western blot (A). While the mRNA of TAZ remained unchanged under USP1 depletion conditions (B). C. USP1 depletion promoted the degradation of TAZ. After treating the OS cells with cycloheximide (CHX) at different time points as indicated, the expression of endogenous TAZ protein was analyzed by Western blot. D. 143B and HOS cells were transfected with Usp1-SiRNA mixture or negative control (SiNC) for 48h, and then MG132 (20 µM, 6 hours) or chloroquine (CQ) (10 µM, 20 hours) were added as indicated. Endogenous ubiquitin, TAZ and LC3 levels were analyzed by Western blot. E. USP1 depletion promoted the ubiquitination of TAZ. 143B and HOS cells were transfected with Usp1-SiRNA mixture or negative control (SiNC) for 48h. Cell lysates was immunoprecipitated with the anti-TAZ antibody and TAZ ubiquitination was then examined by Western blot. F, G. USP1 depletion specifically increased the K11and K29-linked ubiquitination of TAZ, as confirmed by Co-IP assays. H, I. The accumulation of TAZ in nucleus were reduced in case of USP1 depletion in OS cells, as confirmed by Immunofluorescence (H) and Western Blot assay (I). J. OS cells were transfected with Usp1-SiRNA mixture or negative control (SiNC) for 48h, then Co-IP assay was performed to detected the interaction of TAZ and TEAD. K. The expression of downstream genes in Hippo signaling pathway was reduced in case of USP1 depletion, as detected by Western Blot. Data represents the means ± SD. The images and data presented were acquired from and represented three independent experiments. P*< 0.05, P** < 0.01, P*** < 0.001 in comparison with the control group.