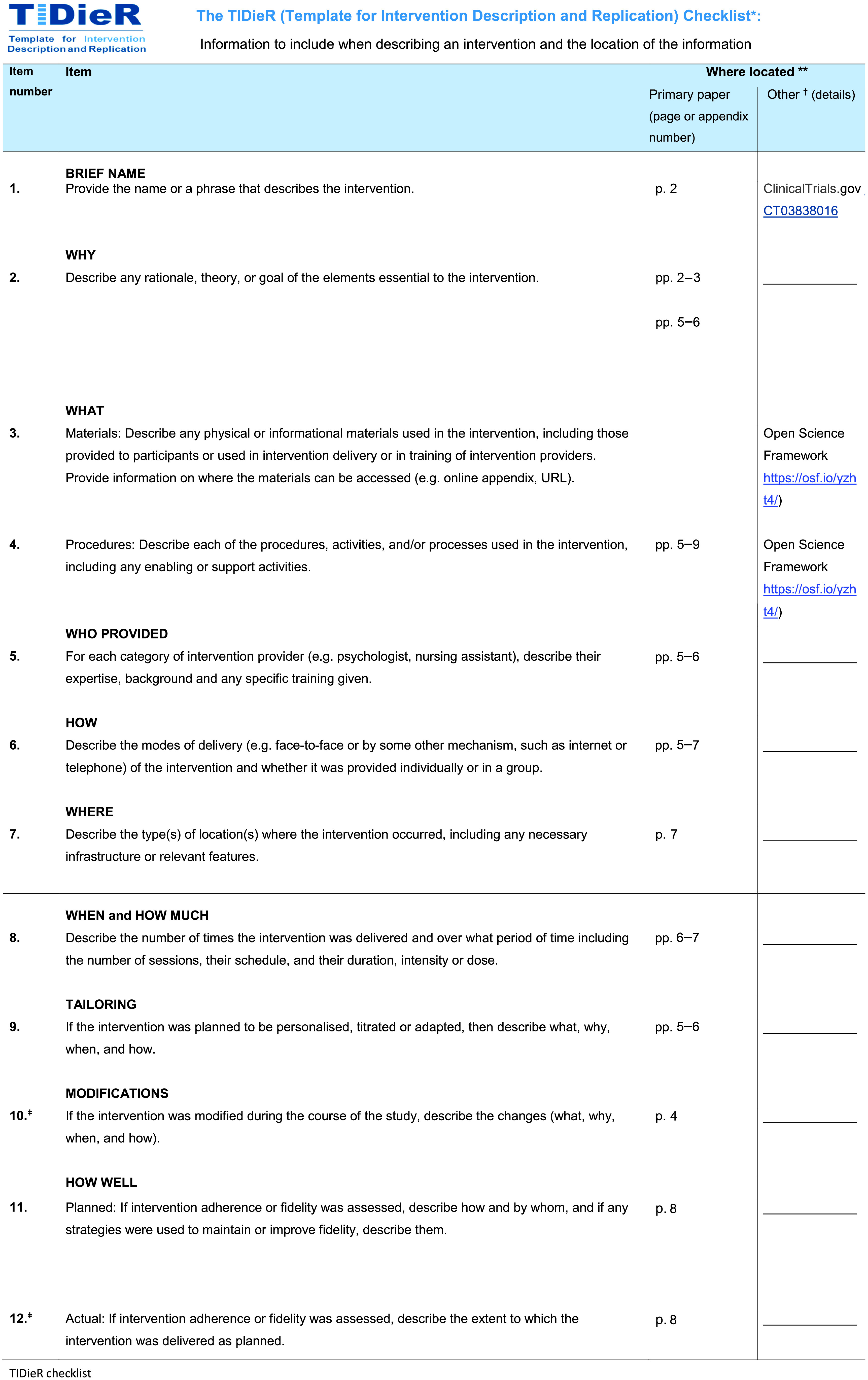
*We strongly recommend using this checklist in conjunction with the TIDieR guide (see BMJ 2014;348:g1687), which contains an explanation and elaboration for each item.
The focus of TIDieR is on reporting details of the intervention elements (and where relevant, comparison elements) of a study. Other elements and methodological features of studies are covered by other reporting statements and checklists and have not been duplicated as part of the TIDieR checklist. When a randomized trial is being reported, the TIDieR checklist should be used in conjunction with the CONSORT statement (see www.consort-statement.org) as an extension of Item 5 of the CONSORT 2010 Statement. When a clinical trial protocol is being reported, the TIDieR checklist should be used in conjunction with the SPIRIT statement as an extension of Item 11 of the SPIRIT 2013 Statement (see www.spirit-statement.org). For alternate study designs, TIDieR can be used in conjunction with the appropriate checklist for that study design (see www.equator-network.org).
**Authors – use N/A if an item is not applicable for the intervention being described. Reviewers – use “?” if information about the element is not reported/not sufficiently reported.
†If the information is not provided in the primary article, give details of where this information is available. This may include locations such as a published protocol or other published articles (provide citation details) or a website (provide the URL).
ǂIf completing the TIDieR checklist for a protocol, these items are not relevant to the protocol and cannot be described until the study is complete.

 This work is licensed under a
This work is licensed under a