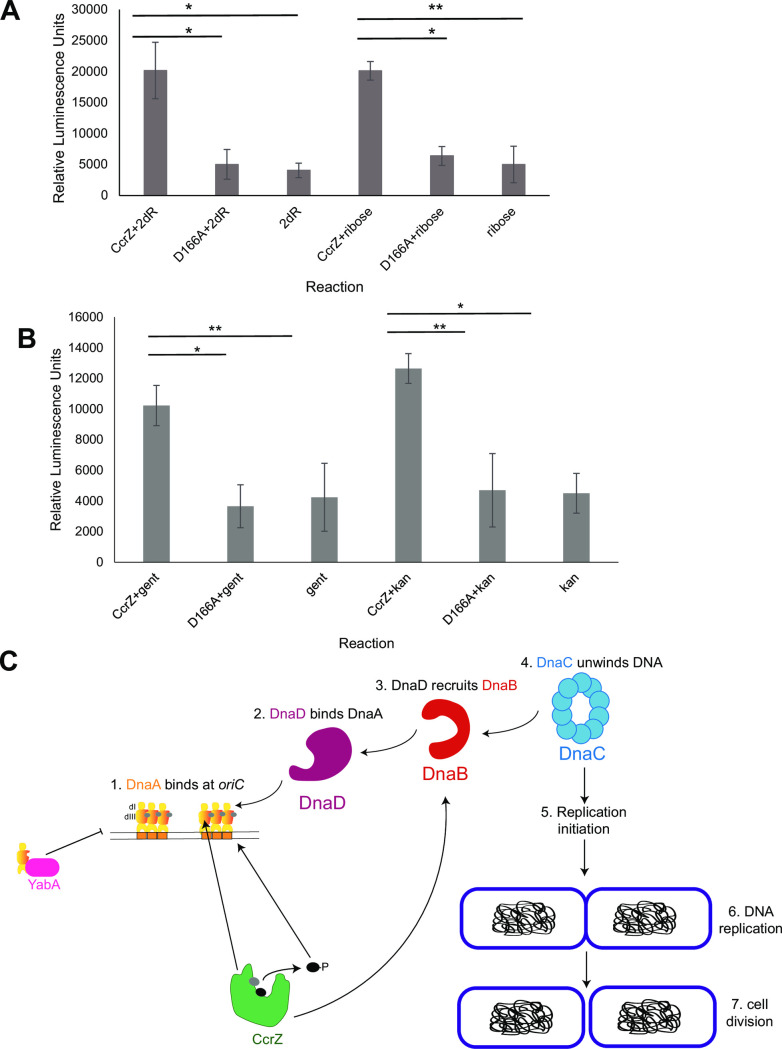
Fig 5. CcrZ demonstrates activity on ribose.
(A) ATPase assay of CcrZ with D-ribose and 2-deoxy-ribose (2dR). Reactions were performed in triplicate, t-tests were used to assess statistical significance as follows (*p = 0.025–0.03, **p = 8.0E-5). Error bars show standard deviation of the mean. (B) ATPase assay of CcrZ with kanamycin and gentamicin. Reactions were also performed in triplicate, with t-tests to assess statistical significance (*p = 0.01–0.029, **p = 4.0–6.0E-4). Error bars represent the standard deviation of the mean. (C) Summary model for CcrZ function in B. subtilis. CcrZ interacts with DnaA domains I, III and DnaB, perhaps to stimulate replication initiation or help localize CcrZ to the origin. We hypothesize that CcrZ contributes to replication initiation through direct protein-protein interactions and through phosphorylation of a second messenger or metabolite. CcrZ phosphorylation of a ribose-like substrate could serve as an upstream signaling molecule to promote replication initiation through oriC. When CcrZ is overexpressed, the increase in phosphorylated substrate and interactions with DnaA and DnaB lead to over-initiation, causing cell elongation and activation of the SOS response.