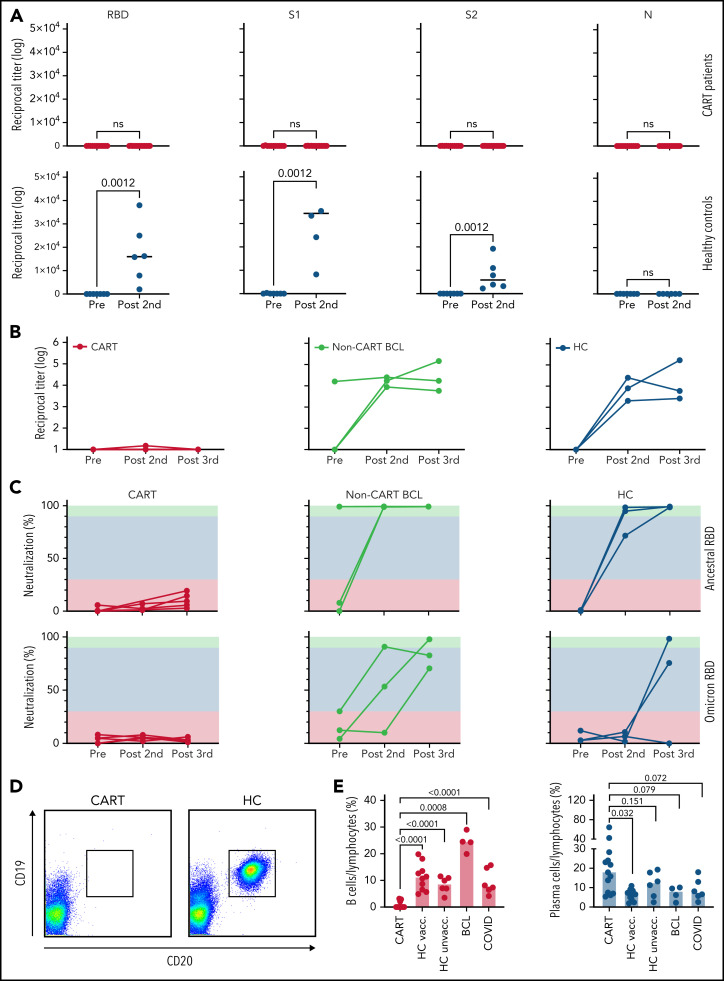
Figure 1.
Anti–SARS-CoV-2 B-cell responses in CART-treated patients after 2 to 3 doses of a COVID-19 mRNA vaccine. (A) Titers of IgG antibodies against different full-length recombinant SARS-CoV-2 proteins were measured in CART-treated patients (top, red) and HCs (bottom, blue) before (11 CART-treated patients, 7 HCs) and after (13 CART-treated patients, 6 HCs) 2 doses of a COVID-19 mRNA vaccine. (B) For 5 CART-treated patients (red), 3 patients with B-cell lymphoma (BCL) without prior CART treatment (yellow), and 3 HCs (blue), samples were available at all 3 time points, and anti-RBD titers were measured before vaccination, after the second dose, and after the third dose. (C) Neutralizing activity before vaccination after the second and third doses of a COVID-19 mRNA vaccine in the peripheral blood of the same groups. Green, orange, and red areas indicate different degrees of inhibition (green, >90%; orange, 30%-89%; red, <30%). Neutralizing activity is shown for both the original ancestral SARS-CoV-2 RBD protein (top) and for its Ο variant (bottom). (D) Example of a flow cytometric analysis of B-cell subpopulations in the peripheral blood of a CART-treated patient (2123-038) and an HC (2123-019; right), before the first dose of the vaccine. Dot plots show CD19+/CD20+ B cells after gating on CD3−/CD56−/CD14− lymphocytes. (E) Percentages of peripheral blood CD19+/CD20+ B cells (red bars) and CD19−/CD38+ cells were determined in 13 CART-treated patients, 10 vaccinated HCs, 4 patients with B-cell lymphoma without prior CART treatment at the prevaccine time point, 6 unvaccinated HCs, and 7 patients with active COVID-19. Data are medians. Differences between groups were analyzed for statistical significance by Mann-Whitney U test.