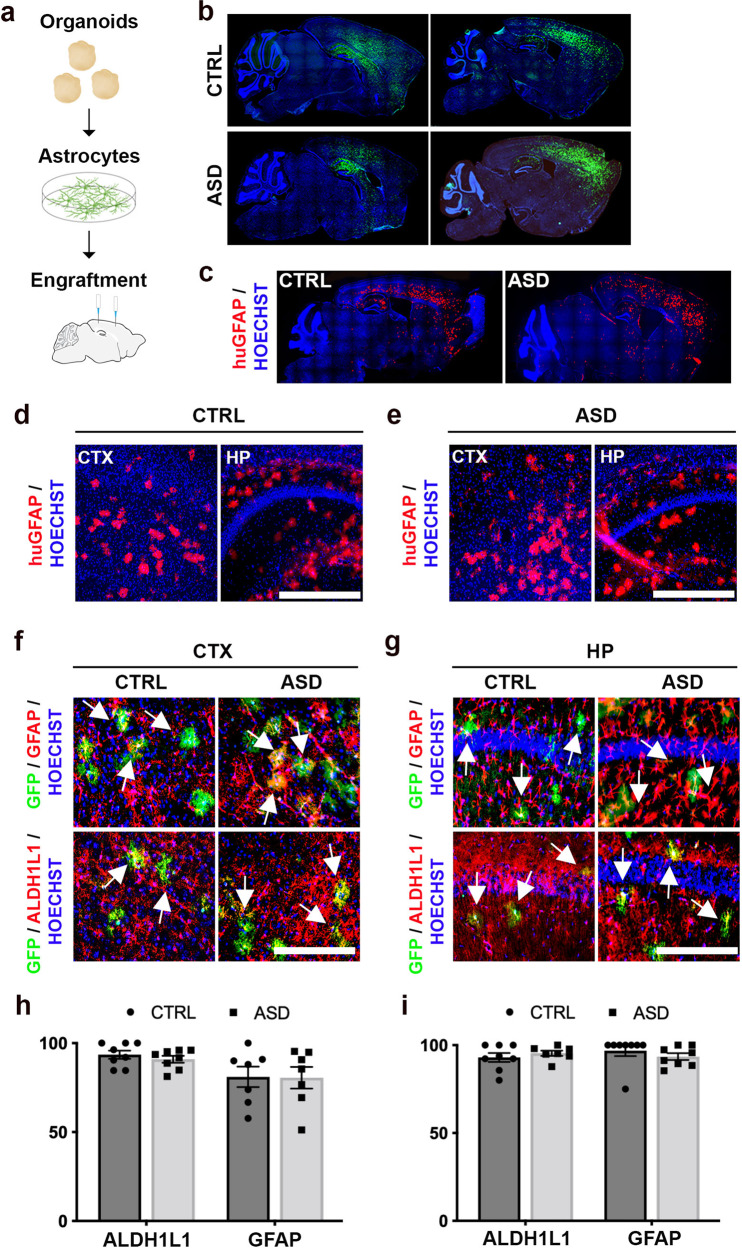
Fig. 2. Organoid-derived astrocytes migrate throughout the mouse cortex and survive into adulthood.
a Schematic of experimental workflow. We transplanted a total of 8–10 × 105 astrocytes (infected with CAG-GFP virus) into the brains of Rag2KO neonatal mice, spread out over four injection sites, which were bilateral along the midline, anterior and posterior to bregma. b–e Immunostained whole brain slices cut on the sagittal plane both for GFP (b) and the human-specific GFAP epitope (huGFAP) at P60 showed a wide and homogenous spread throughout the cortex (c) (see also Supplementary Fig. 4 for stereological quantifications). d, e Representative higher magnification images illustrated the spread of huGFAP in cortex and hippocampus of chimeric mice. f–i Representative co-immunostained images of chimeric brains revealed high co-localization of GFP expression (human astrocytes, green) with the two astrocyte markers, GFAP (red, top panel) and ALDH1L1 (red, bottom) in the cortex (f) and hippocampus (g). Arrows indicate examples of dual positive cells. More than 90% of GFP+ cells expressed astrocyte markers suggesting that astrocytes retained their identities upon maturation in the adult mouse brain (h and i, see also Supplementary Fig. 5). These results establish that CTRL and ASD astrocytes dissociated from organoids generated homogenous transplantations in host brains. CTX Cortex, HP Hippocampus. Scale bar = 500 μm for d and e and 250 μm for f and g. Data are represented as mean ± SEM. Co-localization: CTRL and ASD n = 8 per group (2 mice/2 distinct lines, and 4 slices per brain).