Abstract
Background and aims
Recent media reports of myocarditis after receiving COVID-19 vaccines, particularly the messenger RNA (mRNA) vaccines, are causing public concern. This review summarizes information from published case series and case reports, emphasizing patient and disease characteristics, investigation, and clinical outcomes, to provide a comprehensive picture of the condition.
Methods
A systematic literature search of PubMed and Google scholar was conducted from inception to April 27, 2022. Individuals who develop myocarditis after receiving the COVID-19 vaccine, regardless of the type of vaccine and dose, were included in the study.
Results
Sixty-two studies, including 218 cases, participated in the current systematic review. The median age was 29.2 years; 92.2% were male and 7.8% were female. 72.4% of patients received the Pfizer-BioNTech (BNT162b2) vaccine, 23.8% of patients received the Moderna COVID-19 Vaccine (mRNA-1273), and the rest of the 3.5% received other types of COVID-19 vaccine. Furthermore, most myocarditis cases (82.1%) occurred after the second vaccine dose, after a median time interval of 3.5 days. The most frequently reported symptoms were chest pain, myalgia/body aches and fever. Troponin levels were consistently elevated in 98.6% of patients. The admission ECG was abnormal in 88.5% of cases, and the left LVEF was lower than 50% in 21.5% of cases. Most patients (92.6%) resolved symptoms and recovered, and only three patients died.
Conclusion
These findings may help public health policy to consider myocarditis in the context of the benefits of COVID-19 vaccination.
Keywords: COVID-19, Myocarditis, COVID-19 vaccines, mRNA vaccine, Cardiovascular complications
1. Introduction
International efforts to drive vaccinations are critical to restoring health and economic and social recovery as the SARS-CoV-2 coronavirus (COVID-19)-caused pandemic continues [1]. The COVID-19 vaccines developed by Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) were granted emergency approval by the Food and Drug Administration (FDA) of the United States in December 2020. Reports of myocarditis after the COVID-19 vaccination, notably after the messenger RNA (mRNA) vaccines, have recently received widespread media attention, causing widespread concern among the general public [1]. Myocarditis is diagnosed in about ten to twenty people per 100,000 in the general population each year, and it is more common in men and younger age groups [2]. Myocarditis following mRNA COVID-19 vaccination was first reported in Israel in April 2021, and then several case reports and case series were reported around the world.
Specifically, this report examines the current literature on myocarditis following COVID-19 vaccination, summarizing available information from previously published case reports and case series, with a strong attention on reporting patient and disease characteristics, as well as investigation and clinical outcome, in order to provide a comprehensive picture of the condition.
2. Methods
2.1. Review objectives
The main objective is to clarify the potential occurrence of myocarditis associated with COVID-19 vaccination and elaborate on the demographic and clinical characteristics of COVID-19 vaccinated individuals who develop myocarditis and how many cases have been reported in the literature.
2.2. Protocol and registration
The review is written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines for the systematic review of available literature [3]. The protocol of the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with ID CRD42022308997. The AMSTAR-2 checklist was also used to evaluate this study, and it was found to be of high quality [4]. This review article does not require ethics approval.
2.3. Search strategy
A comprehensive search of major electronic databases (PubMed and Google Scholar) was conducted on April 27 10, 2022, to locate all publications. The AND operator was used to connect two of the most important concepts in the search terminology (“COVID-19″ AND “Myocarditis”). (“Myocarditis” and “COVID-19″ OR “SARS-CoV-2″ OR “Coronavirus Disease 2019″ OR “severe acute respiratory syndrome coronavirus 2″ OR “coronavirus infection” OR ″2019-nCoV” AND “vaccine, vaccination, OR vaccine” were used in the search. To make sure the search was completed, we checked the references of all relevant papers.
2.4. Eligibility criteria
All case series and case reports on post-COVID-19 vaccine myocarditis in humans were included. Individuals who develop myocarditis after receiving the COVID-19 vaccine, regardless of the type of vaccine and dose. The references of the relevant articles will also be reviewed for additional articles that meet the inclusion criteria. Narrative and systematic reviews, original and unavailable data papers were excluded from this review. Moreover, articles other than English were excluded in this review.
2.5. Data extraction and selection process
PRISMA 2020 was used to guide every step of the data extraction process from the original source. Two independent authors (SKA and RAE) used the Rayyan website to screen abstracts and full-text articles based on inclusion and exclusion criteria [5]. The discrepancies between the two independent authors were resolved by discussion. Microsoft Excel spreadsheets collected the necessary information from the extracted data. Author names, year of publication, age, gender, type of COVID-19 vaccine, dose, days to symptoms onset, symptoms, troponin level, LVEF 50% or LVEF >50%, ECG, length of hospital stay/days, treatment, and outcomes were extracted from each study.
2.6. Critical appraisal
To assess the quality of all included studies, we used the Joanna Briggs Institute's critical appraisal tool for case series and case reports [6]. Two different authors (SKA and RAE) evaluated each article, each of whom worked independently. Paper evaluation disputes were resolved through discussion. Articles with an average score of 50% or higher were included in the data extraction process. The AMSTAR 2 criteria were used to evaluate the results of our systematic review [4]. The AMSTAR 2 tool assigned a “moderate” rating to the overall quality of our systematic review.
2.7. Data synthesis and analysis
All the articles included in the current systematic review were analyzed, and the data were extracted and pooled. This included (authors' names, year of publication; gender; type of COVID-19 vaccine, dose, days to symptoms onset, troponin level, LVEF below or above 50%, ECG, length of hospital stay/days; treatment and outcomes). We gathered this data from the results of eligibility studies. COVID-19 vaccine recipients who developed myocarditis were included in the study.
3. Results
3.1. Selection of studies
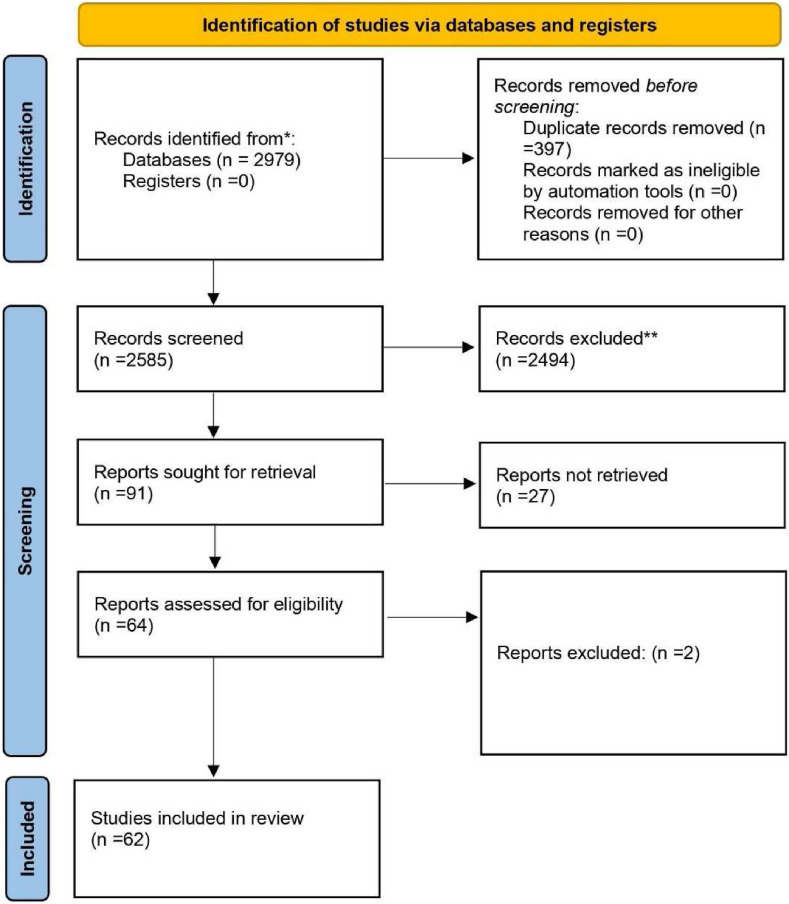
When we searched the major databases (PubMed and Google Scholar) on April 27, 2022, we discovered 2979 articles relevant to our search criteria. A citation manager tool (Mendeley) was then used to organize the references, and 397 articles were automatically removed because they contained duplicate content. Next, the titles, abstracts, and full texts of 2585 articles were checked for accuracy, and 2494 articles were rejected because they did not meet the criteria for inclusion. Besides that, 91 articles were submitted for retrieval, but twenty-seven were rejected because they did not meet our inclusion requirements. The current systematic review was limited to 62 articles in total (Fig. 1 ). The details of case reports and case series are shown in ( Table 1 ).
Fig. 1.
PRISMA flow-diagram.
Table 1.
Characteristics and outcomes of patients with myocarditis related to COVID-19 vaccine.
| Author/Year of publication | Country | Age | Gender | Type of COVID-19 vaccine | Dose | Days to symptom onset | Symptoms | Troponin level | LVEF <50% or LVEF >50% | Electrocardiogram (ECG) | Treatment | Length of hospital stay (days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abu Mouch et al., 2021 [24] | Israel | 6 cases mean age 22 years | All of them were male | BNT162b2 | 2nd in 5 cases and 1st in one case | Mean 4.5 days | Chest pain/discomfort | Elevated in all cases | LVEF >50%in all cases | Abnormal in all cases | NSAIDs and colchicine | Mean 5.6 days | Recovered |
| Marshall et al., 2021 [25] | USA | 7 cases mean age 16.7 years | All of them were male | BNT162b2 | 2nd | Mean 2.57 days | Chest pain | Elevated in all cases | LVEF >50% in 6 cases and LVEF <50% in one case | Abnormal in all cases | NSAIDs, IVIg, IV methylprednisolone, PO prednisone, famotidine, aspirin | Mean 11.57 days | Recovered |
| D'Angelo et al., 2021 [26] | Italy | 30 years | Male | BNT162b2 | 1st | 21 days | dyspnea, constrictive retrosternal pain, nausea, and profuse sweating |
Elevated | LVEF >50% | Abnormal | Bisoprolol, aspirin, and prednisolone | 7 days | Recovered |
| Nassar et al., 2021 [27] | USA | 70 years | Female | . Ad26.COV2·S | 1st | 2 days | The patient arrived at the emergency department in severe respiratory distress | Elevated | LVEF >50% | Abnormal | vasopressors and antibiotic therapy | 8 days | Died |
| Kim et al., 2021 [28] | USA | 4 cases mean age 38.25 years | 3 males and 1 female | mRNA-1273 in 2 cases And BNT162b2 in 2 cases |
2nd | Mean 2.75 days | Chest pain | Elevated in all cases | LVEF >50% in 3 cases and LVEF <50% in one case | Abnormal in all cases | Corticosteroids NSAIDs and colchicine | Mean 2.5 days | Recovered |
| Montgomery et al., 2021 [10] | USA | 23 cases mean age 25 years | All of there were male | BNT162b2 in 7 cases and 16 cases mRNA-1273 | 2nd in 20 cases and 1st in 3 cases | Mean 2 days | Chest pain | Elevated in all cases | LVEF <50% in 4 cases and LVEF ≥50% in 19 cases | Abnormal in 19 cases and normal in 4 cases | All patients received brief supportive care | Mean 7 days | 16 cases were fully recovered and 7 cases under follow-up |
| Verma et al., 2021 [29] | USA | 2 cases (45, 42) years Mean age 43.5 years |
1 male and 1 female | BNT162b2- in 1 case and 1 case mRNA-1273 | 1st in one case and 2nd in another case | Mean 12 days | Chest pain, dyspnea and dizziness, | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | intravenous diuretics, methylprednisolone, lisinopril, spironolactone, and metoprolol succinate). |
7 days | female case recovered and male case died |
| Rosner et al., 2021 [30] | USA | 7 cases Mean age 27.42 years |
All of them were male | BNT162b2 in 5 cases, one case mRNA-1273 and one case Ad26.COV2·S | 2nd in 6 cases and 1st in one case | Mean 3.85 days | Chest pain | Elevated in all cases | LVEF >50% in 6 cases and LVEF <50% in one case | Abnormal in 6 cases and normal in one case | β-blocker and anti-inflammatory medication | Mean 2.85 days | Recovered |
| Dionne et al., 2021 [31] | USA | 15 cases mean age 15 years | 14 cases male and one case female | BNT162b2 in all cases | 2nd in all cases | Mean 3 days | Chest pain, fever, myalgia, headache | Elevated in all cases | Mean LVEF <50% in all case | Abnormal in all cases | β-blocker therapy. | Mean 2 days | Recovered |
| Garcı'a et al., 2021 [32] | Mexico | 39 years | Male | BNT162b2 | 2nd | ¼ day | Chest pain | Elevated | LVEF >50% | Abnormal | anti-inflammatory medication |
6 days | Recovered |
| Dickey et al., 2021 [33] | USA | 6 cases mean age 27 years | All of them were male | BNT162b2 in 5 cases and one case mRNA-1273 | 2nd | Mean 3.33 days | chest pain, chills, myalgia, malaise, headache and fever |
Elevated in all cases | LVEF >50% in 3 cases and LVEF <50% in 3 cases | Abnormal in 5 cases and normal in one case | Unknown | Unknown | Recovered |
| Tano et al., 2021 [34] | USA | 8 cases mean age 16.61 years | All of them were male | BNT162b2 in all cases | 2nd in 7 cases and 1st in one case | Mean 2. 37 days | Chest pain, fatigue, abdominal pain, fever, shortness of breath | Elevated in all cases | LVEF >50% in all cases | Abnormal in 6 cases and normal in 2 cases | NSAIDs | Mean 2.36 days | Recovered |
| Larson et al., 2021 [35] | USA and Italy | 8 cases mean age 31. 62 years | All of them were male | BNT162b2 in 5 cases and 3 cases mRNA-1273 | 2nd in 7 cases and 1st in one case | Mean 2. 75 days | Chest pain, myalgia, fever, chills, shortness of breath and cough | Elevated in all cases | LVEF >50% in 6 cases and LVEF <50% in 2 cases | Abnormal in 7 cases and normal in 1 case | NSAIDs, colchicine and prednisone | Unknown | Recovered |
| Deb et al., 2021 [36] | USA | 67 years | Male | mRNA-1273 | 2nd | ¼ day | Nausea, orthopnea, fatigue | Elevated | LVEF >50% | Normal | intravenous furosemide, bronchodilators | 2 days | Recovered |
| Abbate et al., 2021 [37] | USA | 2 cases mean age 30.5 years | One male and one female | BNT162b2 in all cases | 2nd in in one case and 1st in second case | Mean 5.5 days | Fever, cough, chest pain, nausea and vomiting | Unknown | LVEF <50% in in all cases | Abnormal in all cases | Prednisone | 73 days for one case | One case died and one recovered |
| Muthukumar et al., 2021 [38] | USA | 52 years | Male | mRNA-1273 | 2nd | 1 day | Chest pain, fevers, shaking chills, myalgias, and headache | Elevated | LVEF <50% | Abnormal | low-dose lisinopril and carvedilol | 4 days | Recovered |
| Isaak et al., 2021 [39] | Germany | 15 years | Male | BNT162b2 | 2nd | 1 day | fever, myalgia | Elevated | LVEF <50% | Abnormal | Unknown | 7 days | Recovered |
| Cereda et al., 2021 [40] | Italy | 12 years | Male | BNT162b2 | 2nd | 1 day | Chest pain | Elevated | LVEF >50% | Abnormal | Bisoprolol and ramipril | 7 days | Recovered |
| Watkins et al., 2021 [41] | USA | 20 years | Male | BNT162b2 | 2nd | 2 days | Chest pain and shortness of breath | Elevated | LVEF >50% | Abnormal | Colchicine, metoprolol, ibuprofen | Unknown | Recovered |
| Chamling et al., 2021 [42] | Germany | 3 cases mean age 37.66 years | 2 males and 1 female | BNT162b2 in 2 cases, and ChAdOx1 nCoV-19 in 1 case | 1st in 2 cases and 2nd in 1 case | Mean 7 days | Chest pain | Elevated in 2 case and not elevated in 1 case | LVEF >50% in all cases | Abnormal in 2 cases and normal on 1 case | Unknown | Unknown | Recovered |
| Mansour et al., 2021 [43] | USA | 2 cases mean age 23 years | 1 male and 1 female | mRNA-1273 in all cases | 2nd in all cases | 1 day | Chest pain, fever, | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | metoprolol | Mean 2 days | Recovered |
| Levin et al., 2021 [44] | Israel | 7 cases mean age 20.42 years | All of them were male | BNT162b2 in all cases | 2nd in all cases | Mean 7 days | Chest pain, fatigue, fever and headache | Elevated in all cases | LVEF >50% in 5 cases LVEF <50% in 2 cases | Abnormal in all cases | Colchicine, Ibuprofen, Bisoprolol, and Ramipril | Mean 2.5 days | Recovered |
| Schauer et al., 2021 [45] | USA | 13 cases mean age 15.07 years | 12 male and 1 female | BNT162b2 in all cases | 2nd in all cases | Mean 2.76 days | chest pain, shortness of breath, fever and myalgia | Elevated in all cases | LVEF >50% in 11 cases LVEF <50% in 2 cases | Abnormal 1n 9 cases and normal in 4 cases | NSAIDs | Mean 2 days | Recovered |
| Shumkova et al., 2021 [46] | Poland | 23 years | Male | BNT162b2 | 2nd | 1 day | chest pain, shortness of breath and fever | Elevated | LVEF >50% | Abnormal | Aspirin and methylprednisolone | 6 days | Recovered |
| Minocha et al., 2021 [47] | USA | 17 years | Male | BNT162b2 | 2nd | 2 days | chest pain | Elevated | LVEF >50% | Abnormal | NSAIDs | 6 days | Recovered |
| Hasnie et al., 2021 [48] | USA | 22 years | Male | mRNA-1273 | 1st | 3 days | Chest pain | Elevated | LVEF >50% | Abnormal | Aspirin and colchicine | 2 days | Recovered |
| Starekova et al., 2021 [49] | USA | 5 cases mean age 25.2 years | 4 males and 1 female | BNT162b2 in 3 cases and mRNA-1273 in 2 cases | 2nd in all cases | Mean 2,6 days | Chest pain, fatigue, nausea, fever, chills and myalgia | Elevated in all cases | LVEF >50% in 4 cases LVEF <50% in 1 case | Abnormal in 4 cases and normal in 1 case | Unknown | Unknown | Recovered |
| Koizumi et al., 2021 [50] | Japan | 2 cases mean age 24.5 years | All of them were male | mRNA-1273 in all cases | 2nd in all cases | Mean 2.5 days | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | NSAIDs | Mean 4 days | Recovered |
| McLean et al., 2021 [51] | USA | 16 years | Male | BNT162b2 | 2nd | 1 day | Chest pain | Elevated | LVEF >50% | Abnormal | NSAIDs | 7 days | Recovered |
| Riedel et al., 2021 [52] | Brazil | 47 years | Male | Sinovac COVID-19 vaccine in activated (China) | 2nd | Unknown | Chest pain Cough and myalgia | Elevated | LVEF <50% | Abnormal | Unknown | Unknown | Recovered |
| In-Cheol et al., 2021 [53] | Korea | 24 years | Male | BNT162b2 | 2nd | 1 day | Chest pain | Elevated | LVEF >50% | Abnormal | Unknown | 5 days | Recovered |
| Nguyen et al., 2021 [54] | Germany | 20 years | Male | mRNA-1273 | 1st | 1 day | Chest pain, fatigue and myalgia | Elevated | LVEF >50% | Abnormal | Unknown | Unknown | Recovered |
| Azdaki et al., 2021 [55] | Iran | 70 years | Male | ChAdOx1 nCoV-19. | 1st | 3 days | Syncope | Elevated | LVEF >50% | Abnormal | magnesium sulfate | 7 days | Recovered |
| Sokolska et al., 2021 [56] | Poland | 21 years | Male | BNT162b2 | 1st | 3 days | Chest pain | Elevated | LVEF >50% | Abnormal | Unknown | Unknown | Recovered |
| Patel et al., 2021 [57] | USA | 5 cases mean age 23.2 years | All of them were male | BNT162b2 in 4 cases and mRNA-1273 in 1 case | 2nd in 4 cases and 1st in 1 case | Mean 2.2 days | Chest pain, dyspena, nausea, headache and chills | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | Colchicine, Ibuprofen and aspirin | Mean 1.8 days | Recovered |
| Kim et al., 2021 [58] | Korea | 29 years | Male | BNT162b2 | 2nd | 1 day | Chest pain | Elevated | LVEF >50% | Normal | corticosteroids and NSAIDs. | 7 days | Recovered |
| Ehrlich et al., 2021 [59] | Germany | 40 years | Male | BNT162b2 | 1st | 6 days | chest pain and shortness of breath, and fever | Elevated | LVEF <50% | Abnormal | Aspirin, heparin, beta-blocker and a mineralocorticoid antagonist | 2 days | Recovered |
| Schmitt et al., 2021 [60] | France | 19 years | Male | BNT162b2 | 2nd | 3 days | Chest pain and dyspnea | Elevated | LVEF >50% | Abnormal | Unknown | 1 day | Recovered |
| Kadwalwala et al., 2021 [61] | USA | 38 years | Male | mRNA-1273 | 1st | 2 days | Chest pain, fatigue and fever | Elevated | LVEF <50% | Abnormal | Methylprednisolone, lisinopril, and spironolactone | 6 days | Recovered |
| Azir et al., 2021 [62] | USA | 17 years | Male | BNT162b2 | 2nd | 1 day | Chest pain | Elevated | LVEF >50% | Abnormal | aspirin and sublingual nitroglycerin | 1 day | Recovered |
| Gabriel Amir et al., 2022 [63] | Israel | 15 cases mean age 17.03 years | All of them were male | BNT162b2 in all cases | 2nd in 14 cases and 1st in 1 case | Median 4.7 days | Chest pain and fever | Elevated in all cases | LVEF >50% in 12 cases LVEF <50% in 3 case | Abnormal in 14 cases and normal in 1 case | NSAIDs, colchicine, aspirin | Mean 5 days | Recovered |
| Ahmed SK 2022 [64] | Iraq | 7 cases mean age 24.5 years | All of them were male | BNT162b2 in 5 cases and mRNA-1273 in 2 cases | 2nd in all cases | Median 2.14 days | Chest pain, fever, fatigue, SOB | Elevated in all cases | LVEF >50% in 6 cases LVEF <50% in 1 case | Abnormal in all cases | colchicine and NSAIDs | Mean 2.4 days | Recovered |
| Mateusz Puchalski et al., 2022 [21] | Poland | 5 cases mean age 16.6 years | All of them were male | BNT162b2 in all cases | 2nd in 2 cases and 1st in3 cases | Median 6.4 days | Chest pain, fever, shoulder pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | ACEI | Mean 12.3 days | Recovered |
| Carolyn M. Rosner et al., 2022 [65] | USA | 7 cases mean age 29.14 years | All of them were male | BNT162b2 in 4 cases and mRNA-1273 in 2 cases and J&J in 1 case | 2nd in all cases | Median 3 days | Chest pain, SOB | Elevated in all cases | LVEF >50% in all cases | Abnormal in 6 cases and normal in 1 case | NA | NA | Recovered |
| Agata Łaźniak-Pfajfer1 et al., 2022 [66] | Poland | 3 cases mean age 17 years | All of them were male | BNT162b2 in all cases | 2nd in 1 case and 1st in2 cases | NA | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in 1 case and normal in 2 cases | NA | NA | Recovered |
| Yoshiki Murakami et al., 2022 [67] | Japan | 2 cases mean age 32.5 | All of them were male | BNT162b2 in all cases | 2nd in 1 case and 1st in 1 case | Median 6.5 days | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in 1 case and normal in 1 case | Colchicine, NAIADs | Mean 5.5. days | Recovered |
| Farah Naghashzadeh et al., 2022 [68] | Iran | 1 case 29years | Female | rAd26 and rAd5 (Sputnik V vaccine) | 2nd | 2 days | Chet pain | Elevated | LVEF <50% | Abnormal | methylprednisolone, prednisolone, and mycophenolate mofetil |
7 days | Recovered |
| Chan-Hee Lee et al., 2022 [69] | South Korea | 1 case 22 years | Male | mRNA-1273 | 2nd | 5 days | Chest pain | Elevated | LVEF >50% | Abnormal | NAIADs | 5 days | Recovered |
| Xavier Fosch et al., 2022 [70] | Spain | 24 years | Male | BNT162b2 | 3rd | 2 days | Chest pain | Elevated | LVEF >50% | Abnormal | NAIADs, colchicine | NA | Recovered |
| Daniel A. Gomes et al., 2022 [71] | Portugal | 32 years | Male | mRNA-1273 | 2nd | 2 days | Chest pain | Elevated | NA | Abnormal | NA | NA | Recovered |
| Eduardo Terán Brage et al., 2022 [72] | Spain | 62 years | Female | mRNA-1273 | 3rd | 1 day | Fever | Elevated | LVEF >50% | Abnormal | NSAIDs and colchicine | 3 days | Recovered |
| Arman Sharbatdaran et al., 2022 [73] | USA | 25 years | Male | mRNA-1273 | 2nd | 3 days | shortness of breath, headache, fever, and sweating | Elevated | NA | Abnormal | Colchicine, aspirin | 5 days | Recovered |
| Julia Moosmann et al., 2022 [74] | New Zealand | 2 cases mean age 13 years | Male | BNT162b2 in all cases | 2nd in all cases | Median 2.5 days | Chest pain | Elevated in all cases | LVEF >50% | Abnormal in all cases | NA | Median 7.5 days | Recovered |
| Carlotta Sciaccaluga et al., 2022 [75] | Italy | 2 cases men age 20.5 years | All of them were male | mRNA-1273 | 2nd in all cases | Median 3 days | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | beta-blockers,antagonists, NSAIDs | Median 9 days | Recovered |
| Arianne Clare C. Agdamag et al., 2022 [76] | USA | 80 years | Female | BNT162b2 | 1st | 12 days | nausea, emesis, and diarrhoea. | Elevated | LVEF <50% | Abnormal | Methylprednisolone, metoprolol succinate, spironolactone | 14 days | Recovered |
| Samuel Nunn et al., 2022 [77] | Germany | 4 cases mean age 29.5 years | 3 cases were male and 1 case were female | BNT162b2 in 3 cases and mRNA-1273 in 1 case | 2nd in 3 cases and 1st in 1 case | Median 7.5 days | Chest pain, | Elevated in all cases | LVEF >50% in all cases | Abnormal in all cases | NA | Median 3 days | Recovered |
| Kanak Parmar et al., 2022 [78] | USA | 4 cases mean age 29 years | 3 cases were male and 1 case were female | mRNA-1273 in all cases | 2nd in 3 cases and 1st in 1 case | Median 4 days | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in 3 cases and normal in 1 case | Methylprednisolone | Median 7.5 days | Recovered |
| Mohammad Dlewati et al., 2022 [79] | USA | 48 years | Male | mRNA-1273 | 2nd | 2 days | Chest pain | Elevated | LVEF <50% | Abnormal | Metoprolol succinate, ramipril, atorvastatin | 2 days | Recovered |
| Nobuko Kojima et al., 2022 [80] | Japan | 17 years | Male | BNT162b2 | 2nd | 2 days | Chest pain | Elevated | LVEF >50% | Abnormal | Aspirin, colchicine | 23 days | Recovered |
| Katie A. Sharff et al., 2022 [81] | USA | 6 cases mean age 28.8 years | 4 cases were male and 2 cases were female | BNT162b2 in 5 cases and J&J in 1 case | 3rd in 5 cases and 2nd in 1 case | Median 5.6 days | Chest pain | Elevated in 5 cases and normal in 1 case | LVEF >50% in 5 cases and LVEF <50% in 1 case | Abnormal in all cases | NA | Median 1.5 days | NA |
| Suresh Babu Chellapandian et al., 2022 [82] | Turkey | 22 years | Male | mRNA-1273 | 2nd | 2 days | Chest pain | Elevated | NA | Normal | colchicine | 2 days | Recovered |
| Arthur Shiyovich et al., 2022 [19] | Israel | 4 cases mean 31 years | 3 cases were male and 1 case were female | BNT162b2 in all cases | 3rd in all cases | Median 5.7 days | Chest pain | Elevated in all cases | LVEF >50% in all cases | Abnormal in 3 cases and normal in 1 case | NA | NA | Recovered |
3.2. Characteristics of the included studies
Overall, sixty-two studies, including 218 cases each, from the United States, Italy, Israel, Germany, Poland, France, Korea, Brazil, Japan, Mexico, Spain, New Zealand, Portugal, Germany, Iraq Turkey and Iran participated in this systematic review. The median age was 29.2 years; 92.2% were male and 7.8% were female. 72.4% of patients received the Pfizer-BioNTech (BNT162b2) vaccine, 23.8% of patients received the Moderna COVID-19 Vaccine (mRNA-1273), and the rest of the 3.5% received other types of vaccines (Johnson & Johnson, AstraZeneca, Sinovac, Sputnik V vaccine).
The vast majority of cases are from the United States. All patients were diagnosed with myocarditis or myopericarditis following COVID-19 vaccination, regardless of the type of vaccine and dose.
Furthermore, most myocarditis cases (82.1%, n = 179) occurred after the second vaccine dose, after a median time interval of 3.5 days. The most frequently reported symptoms were chest pain (99.1% n = 216), fever (31.6% n = 69), myalgia/body aches (36.6% n = 80), and also variable reports of viral prodromes such as chills, headaches, and malaise. Troponin levels were consistently elevated in 98.6% (n = 215) of the cases where they were reported, consistent with myocardial injury. The admission electrocardiogram (ECG) was abnormal in 88.5% (n = 193) of cases, and the left ventricular ejection fraction (LVEF) was lower than 50% in 21.5% (n = 47) of cases. The median length of hospital stay was 5.8 days in 182 patients but unknown in 36 patients. The vast majority of patients (92.6%) (n = 202) resolved symptoms and recovered, and only three patients died (Table 2 ).
Table 2.
Summary of pooled data from included research papers have been reported in the literature (n = 218).
| Age | Median age - 29.23 years |
|---|---|
| Gender (n) % | Male – 201 (92.2%) |
| Female – 17 (7.8%) | |
| Type of COVID-19 vaccine (n) % | Pfizer-BioNTech (BNT162b2) – 158 (72.4%) |
| Moderna COVID-19 Vaccine (mRNA-1273) - 52 (23.8%) | |
| Janssen (Johnson & Johnson) (Ad26.COV2. S) – 4 (1.8%) | |
| Oxford, AstraZeneca COVID-19 vaccine ChAdOx1 nCoV-19 – 2 (0.9%) | |
| Sinovac COVID-19 vaccine in activated – 1 (0.4%) | |
| rAd26 and rAd5 (Sputnik V vaccine) – 1 (0.4%) | |
| Dose (n) % | First dose – 28 (12.8%) |
| Second dose – 179 (82.1%) | |
| Third dose – 11 (5.1%) | |
| Days to symptom onset | Median 3.57 days |
| Symptoms (n) % | Chest pain - 216 (99.1%) |
| Fever – 69 (31.6%) | |
| Myalgia/body aches – 80 (36.6%) | |
| Shortness of breath – 19 (8.7%) | |
| Troponin level (n) % | Elevated – 215 (98.6%) |
| Not elevated – 2 (0.9%) | |
| Unknown – 1 (0.4%) | |
| LVEF (n) % | LVEF >50% - 169 (77.5%) |
| LVEF <50% - 47 (21.5%) | |
| Unknown – 2 (0.9%) | |
| Electrocardiogram (ECG) (n) % | Abnormal – 193 (88.5%) |
| Normal – 25 (11.5%) | |
| Length of hospital stay (days) | Median 5.8 days in 182 patients |
| Unknown in 36 patients | |
| Outcome (n) % | Recovered – 202 (92.6%) |
| Under follow-up – 7 (3.2%) | |
| Unknown – 7 (2.7%) | |
| Died – 3 (1.4%) |
4. Discussion
The current systematic review summarized evidence from the original case reports and case series that explored the development of myocarditis after the COVID-19 vaccination. Throughout the selected studies, most of the participants were male, from the USA, and their mean age 29.2 was years old. The vaccine-induced myocarditis mechanism is unknown but may be related to the active pathogenic component of the vaccine and specific human proteins, which could lead to immune cross-reactivity resulting in autoimmune disease, which is one cause of myocarditis [[7], [8], [9], [10]]. The occurrence of myocarditis in men may be related to sex hormone variations, as testosterone hormone suppresses anti-inflammatory immune cells while promoting more aggressive T helper cells [7,11].
These findings were matched with Oster et al. (2022) [12], who found the incidence rate of myocarditis among vaccinated male people was similar to that seen in typical cases of myocarditis and there was a strong male predominance for both conditions [13]. Fatima et al. (2022) [7] found most patients who developed myocarditis were males. Moreover, Patone et al. (2022) [14] mentioned that the incidence of myocarditis was among England males younger than 40 years old. Similarly, a systematic review study found that the Incidence of myocarditis following mRNA vaccines is low but probably highest in males aged 12–29 years old [15].
Another important finding in the current systematic review is that most participants received Pfizer-BioNTech (BNT 162b2) followed by the Moderna CVID-19 vaccine (mRNA-1273), and most of the cases who complained of myocarditis received two doses of the vaccine. This indicates that mRNA vaccines are associated with a higher risk of developing myocarditis than viral vector vaccines, including Janssen, Oxford, and Sinovac. Bozkurt et al. (2021) [2], have assumed that autoantibody generation could attack cardiac myocytes in response to the mRNA vaccine, increasing the risk.
Oster et al. (2022) [12] concluded that the risk of myocarditis after the mRNA vaccine was increased after the second dose in adolescents and young males. This finding is matched with Patone (2022) [14], who mentioned the risk of myocarditis increased within a week of receiving the first dose of both adenovirus and mRNA vaccines and after the second dose of mRNA vaccine. On the other hand, Simone et al. (2021) [16] concluded no relationship between COVID-19 mRNA vaccination and post vaccination myocarditis.
The findings extend these observations, including the median onset of symptoms after vaccine administration was 3.5 days. The most common symptoms are chest pain, followed by myalgia/body aches and fever. These findings matched with Pillay et al. (2021) [15], who reported in a systematic review that most myocarditis cases had a short symptoms onset of 2–4 days after a second dose, and the majority presented with chest pain. These findings matched with Oster et al. (2022) [12], who mentioned myocarditis was diagnosed within days of vaccination.
The diagnosis is often established by heart biopsy in patients with severe myocarditis. In patients with mild myocarditis, the diagnosis is based on compatible clinical findings and confirmed by elevated levels of blood markers or an electrocardiogram (ECG) indicative of cardiac injury, with new abnormalities on echocardiography or cardiac MRI [17].
Cardiac-specific investigations revealed that troponin levels were elevated in almost all cases, consistent with myocardial injury, which is associated with autoimmune processes matched with vaccine protein and the case immune system.
In the same lines as Lee et al. (2022) [1], a systematic review to investigate myocarditis following COVID-19 Vaccination in October 2020–October 2021, mentions that all reported cases have an elevated troponin level in keeping with myocardial injury.
In our study, less than one third of cases had left ventricle ejection fraction (LVEF) was less than 50%. Compared to patients with COVID-19 illness, patients with vaccine associated myocarditis had a higher LVEF%.
This finding is consistent with Fronza et al. (2022) [18], who investigated myocardial injury patterns at MRI in COVID-19 Vaccine and discovered that more than half of the cases had more than 50% LVEF. Also, Shiyovich et al. (2022) [19], who analyzed myocarditis following the third (Booster) dose of COVID-19 vaccination, found that the mean left ventricular ejection fraction was 61 ± 7% (range 53–71%). Regional wall motion abnormalities were present in one of the patients only. Global T1 values were increased in one (25%) of the patients, while focal values were increased in 3 (75%) of the patients, Global T2 values were raised in one (25%) of the patients, while focal values were increased in all of the patients (100%). Global ECV was increased in 3 (75%) of the patients, while focal ECV was increased in all the patients (100%). LGE was present in all the patients.
In our systematic review and meta-analysis study, 88.5% of patients had abnormal changes in the electrocardiogram (ECG) result, regardless of the vaccine type.
Vidula et al. (2021) [20] support our findings by reporting two patients with clinically suspected myocarditis who presented with acute substernal chest pain and/or dyspnea after receiving the second dose of the vaccine and were found to have diffuse ST elevations on electrocardiogram (ECG), elevated cardiac biomarkers and inflammatory markers, and mildly reduced left ventricular (LV) function on echocardiography.
Also, Puchalski et al. [21] reported the findings of a case series regarding COVID-19-Vaccination-Induced Myocarditis in Teenagers. Electrocardiogram (ECG) patterns varied, but characteristic features of acute myocardial injury, including ST segment elevation or depression, and repolarization time abnormalities, were present in all cases.
Management of myocarditis remains mainly supportive and is based on restoring hemodynamic stability and the administration of guideline-directed heart failure and arrhythmia treatment. According to our findings, all cases were treated with NSAIDs, beta-blockers, calcium channel blockers, and diuretics. Patients with preserved ventricular function and non-severe features were often treated with colchicine or non-steroidal anti-inflammatory drugs. The median length of hospital stay was 5.28 days in 182 patients, and the vast majority of patients resolved symptoms and recovered, and only 3 patients died.
This finding broadly supports the work of other studies in this area. Woo et al. [22] reported that many patients who received anti-inflammatory agents such as NSAIDs, colchicine, steroids, and intravenous immunoglobulin recovered without further medical treatment, with a hospital stay lasting 3–6 days.
In accordance with the present results, previous studies have demonstrated that almost all of the cases experienced a prompt recovery with no residual cardiac dysfunction. The median length of stay for all myocarditis cases was around 2–3 days, with a range of 2–10 days [23].
5. Conclusion
In conclusion, these findings may help public health policy consider myocarditis in the context of the benefits of COVID-19 vaccination and assess the cardiac condition before the choice of vaccine, which is offered to male adults. In addition, it must be carefully weighed against the very substantial benefit of vaccination. Moreover, further research is required to assess the long-term consequences and other risk factors following immunization, specifically the mRNA vaccines.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author agreement statement
We declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We confirm that all have agreed with the order of authors listed in our manuscript. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions, and final approval of proofs.
Data availability statement
All relevant data are within the manuscript and its supporting information files.
Authors’ contributions
Conception and design SKA acquisition of data SKA, RAE, MGM, EAA analysis and interpretation of data SKA, MGM, RAE, EEA, drafting of the manuscript SKA, RAE MGM, EAA critical revision of the manuscript for important intellectual content statistical analysis SKA, MGM, RAE, EEA, PKI, AAK, ZHW administrative SKA, technical SKA, PKI, AAK, ZHW, supervision SKA, and all authors approving the final draft.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
There is no conflict to be declared.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2022.102513.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee A.S.Y., Iswaree D.D., Balakrishnan O., Khoo C.Y., Ng C.T., Loh J.K.X., et al. Myocarditis following COVID-19 vaccination: a systematic review (october 2020–october 2021) Heart Lung Circ. 2022:S1443–S9506. doi: 10.1016/j.hlc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 4.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. vol. 5. Joanna Briggs Inst Rev Manual Joanna Briggs Inst; 2017. https://synthesismanual.jbi.global/ (Chapter 7: systematic reviews of etiology and risk). [Google Scholar]
- 7.Fatima M., Cheema H.A., Khan M.H.A., Shahid H., Ali M.S., Hassan U., et al. Development of myocarditis and pericarditis after COVID-19 vaccination in adult population: a systematic review. Ann Med Surg. 2022 doi: 10.1016/j.amsu.2022.103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 9.Su J.R. VAERS); 2021. Myopericarditis following COVID-19 vaccination: updates from the vaccine adverse event reporting system. [Google Scholar]
- 10.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairweather D., Cooper L.T., Jr., Blauwet L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. doi: 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from december 2020 to august 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kytö V., Sipilä J., Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013;99:1681–1684. doi: 10.1136/heartjnl-2013-304449. [DOI] [PubMed] [Google Scholar]
- 14.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021:1–13. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillay J., Bialy L., Gaudet L., Wingert A., Mackie A., Paterson D.I., et al. Myocarditis and pericarditis following COVID-19 vaccination: rapid systematic review of incidence, risk factors, and clinical course. medRxiv. 2021 doi: 10.1136/bmj-2021-069445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simone A., Herald J., Chen A., Gulati N., Shen A.Y.-J., Lewin B., et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2021:1–3. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fronza M., Thavendiranathan P., Chan V., Karur G.R., Udell J.A., Wald R.M., et al. Myocardial injury pattern at MRI in COVID-19 vaccine–associated myocarditis. Radiology. 2022 doi: 10.1148/radiol.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiyovich A., Witberg G., Aviv Y., Kornowski R., Hamdan A. A case series of myocarditis following third (booster) dose of COVID-19 vaccination: magnetic resonance imaging study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.839090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidula M.K., Ambrose M., Glassberg H., Chokshi N., Chen T., Ferrari V.A., et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus. 2021:13. doi: 10.7759/cureus.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puchalski M., Kamińska H., Bartoszek M., Brzewski M., Werner B. COVID-19-Vaccination-Induced myocarditis in teenagers: case series with further follow-up. Int J Environ Res Publ Health. 2022;19:3456. doi: 10.3390/ijerph19063456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo W., Kim A.Y., Yon D.K., Lee S.W., Hwang J., Jacob L., et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID-19 vaccine. J Med Virol. 2021 doi: 10.1002/jmv.27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almas T., Rehman S., Mansour E., Khedro T., Alansari A., Malik J., et al. Epidemiology, clinical ramifications, and cellular pathogenesis of COVID-19 mRNA-vaccination-induced adverse cardiovascular outcomes: a state-of-the-heart review. Biomed Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouch S.A., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021:148. doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 26.D'Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassar M., Nso N., Gonzalez C., Lakhdar S., Alshamam M., Elshafey M., et al. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metabol Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma A.K., Lavine K.J., Lin C.-Y. Myocarditis after covid-19 mRNA vaccination. N Engl J Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García B., Ortega P., Ja B.F., León C., Burgos R., Dorta C. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol. 2021;74:812–814. doi: 10.1016/j.rec.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickey J.B., Albert E., Badr M., Laraja K.M., Sena L.M., Gerson D.S., et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. Cardiovasc Imaging. 2021;14:1862–1863. doi: 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tano E., San Martin S., Girgis S., Martinez-Fernandez Y., Sanchez Vegas C. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J Pediatric Infect Dis Soc. 2021;10:962–966. doi: 10.1093/jpids/piab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson K.F., Ammirati E., Adler E.D., Cooper L.T., Jr., Hong K.N., Saponara G., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deb A., Abdelmalek J., Iwuji K., Nugent K. Acute myocardial injury following COVID-19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211029230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbate A., Gavin J., Madanchi N., Kim C., Shah P.R., Klein K., et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int J Cardiol. 2021;340:119–121. doi: 10.1016/j.ijcard.2021.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthukumar A., Narasimhan M., Li Q.-Z., Mahimainathan L., Hitto I., Fuda F., et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaak A., Feisst A., Luetkens J.A. Myocarditis following COVID-19 vaccination. Radiology. 2021;301:E378–E379. doi: 10.1148/radiol.2021211766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cereda A., Conca C., Barbieri L., Ferrante G., Tumminello G., Lucreziotti S., et al. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or atypical causal relationship? Anatol J Cardiol. 2021;25:522. doi: 10.5152/AnatolJCardiol.2021.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins K., Griffin G., Septaric K., Simon E.L. Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med. 2021;50:815–e1. doi: 10.1016/j.ajem.2021.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamling B., Vehof V., Drakos S., Weil M., Stalling P., Vahlhaus C., et al. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clin Res Cardiol. 2021;110:1850–1854. doi: 10.1007/s00392-021-01916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansour J., Short R.G., Bhalla S., Woodard P.K., Verma A., Robinson X., et al. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imag. 2021;78:247–249. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin D., Shimon G., Fadlon-Derai M., Gershovitz L., Shovali A., Sebbag A., et al. Myocarditis following COVID-19 vaccination–a case series. Vaccine. 2021;39:6195–6200. doi: 10.1016/j.vaccine.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schauer J., Buddhe S., Colyer J., Sagiv E., Law Y., Chikkabyrappa S.M., et al. Myopericarditis after the Pfizer messenger ribonucleic acid coronavirus disease vaccine in adolescents. J Pediatr. 2021;238:317–320. doi: 10.1016/j.jpeds.2021.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shumkova M., Vassilev D., Karamfiloff K., Ivanova R., Stoyanova K., Yaneva-Sirakova T., et al. Acute myocarditis associated with the Pfizer/BioNTech vaccine. Kardiol Pol. 2021;79:1282–1283. doi: 10.33963/KP.a2021.0095. [DOI] [PubMed] [Google Scholar]
- 47.Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasnie A.A., Hasnie U.A., Patel N., Aziz M.U., Xie M., Lloyd S.G., et al. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord. 2021;21:1–6. doi: 10.1186/s12872-021-02183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starekova J., Bluemke D.A., Bradham W.S., Grist T.M., Schiebler M.L., Reeder S.B. Myocarditis associated with mRNA COVID-19 vaccination. Radiology. 2021;301:E409–E411. doi: 10.1148/radiol.2021211430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koizumi T., Awaya T., Yoshioka K., Kitano S., Hayama H., Amemiya K., et al. Myocarditis after COVID-19 mRNA vaccines. QJM An Int J Med. 2021;114:741–743. doi: 10.1093/qjmed/hcab244. [DOI] [PubMed] [Google Scholar]
- 51.McLean K., Johnson T.J. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med. 2021;28:918–921. doi: 10.1111/acem.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riedel P.G., Sakai V.F., Toniasso S. de CC., Brum M.C.B., Fernandes F.S., Pereira R.M., et al. Heart failure secondary to myocarditis after SARS-CoV-2 reinfection: a case report. Int J Infect Dis. 2021;113:175–177. doi: 10.1016/j.ijid.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.In-Cheol K., Hyungseop K., Jeong L.H., Yoon K.J., Jin-Young K. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J Kor Med Sci. 2021;36:1–6. doi: 10.3346/jkms.2021.36.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen T.D., Mall G., Westphal J.G., Weingärtner O., Möbius-Winkler S., Schulze P.C. ESC Hear Fail; 2021. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azdaki N., Farzad M. Long QT interval and syncope after a single dose of COVID-19 vaccination: a case report. Pan Afr Med J. 2021:40. doi: 10.11604/pamj.2021.40.67.31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sokolska J.M., Kurcz J., Kosmala W. Every rose has its thorns—acute myocarditis following COVID-19 vaccination. Kardiol Pol. 2021;79:1153–1154. doi: 10.33963/KP.a2021.0075. [DOI] [PubMed] [Google Scholar]
- 57.Patel Y.R., Louis D.W., Atalay M., Agarwal S., Shah N.R. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series. J Cardiovasc Magn Reson. 2021;23:1–8. doi: 10.1186/s12968-021-00795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D., Choi J.H., Jang J.Y., So O., Cho E., Choi H., et al. A case report for myopericarditis after BNT162b2 COVID-19 mRNA vaccination in a Korean young male. J Kor Med Sci. 2021:36. doi: 10.3346/jkms.2021.36.e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlich P., Klingel K., Ohlmann-Knafo S., Hüttinger S., Sood N., Pickuth D., et al. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: case report. Clin Res Cardiol. 2021;110:1855–1859. doi: 10.1007/s00392-021-01936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt P., Demoulin R., Poyet R., Capilla E., Rohel G., Pons F., et al. Acute Myocarditis after COVID-19 vaccination: a case report. Rev Med Interne. 2021;42:797–800. doi: 10.1016/j.revmed.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kadwalwala M., Chadha B., Ortoleva J., Joyce M. Multimodality imaging and histopathology in a young man presenting with fulminant lymphocytic myocarditis and cardiogenic shock after mRNA-1273 vaccination. BMJ Case Reports CP. 2021;14 doi: 10.1136/bcr-2021-246059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azir M., Inman B., Webb J., Tannenbaum L. STEMI mimic: focal myocarditis in an adolescent patient After mRNA COVID-19 vaccine. J Emerg Med. 2021;61:e129–e132. doi: 10.1016/j.jemermed.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amir G., Rotstein A., Razon Y., Beyersdorf G.B., Barak, Corren Y., Godfrey M.E., et al. CMR imaging 6 months after myocarditis associated with the BNT162b2 mRNA COVID-19 vaccine. Pediatr Cardiol. 2022:1–8. doi: 10.1007/s00246-022-02878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed S.K. Myocarditis after BNT162b2 and mRNA-1273 COVID-19 vaccination: a report of 7 cases. Ann Med Surg. 2022 doi: 10.1016/j.amsu.2022.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosner C.M., Atkins M., Saeed I.M., de Lemos J.A., Khera A., Maghsoudi A., et al. Patients with myocarditis associated with COVID-19 vaccination. J Am Coll Cardiol. 2022;79:1317–1319. doi: 10.1016/j.jacc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Łaźniak-Pfajfer A., Surmacz R., Rajewska-Tabor J., Pyda M., Lesiak M., Bobkowski W. Myocarditis associated with COVID-19 vaccination in three teenage males. Pol Arch Intern Med. 2021 doi: 10.20452/pamw.16160. [DOI] [PubMed] [Google Scholar]
- 67.Murakami Y., Shinohara M., Oka Y., Wada R., Noike R., Ohara H., et al. Myocarditis following a COVID-19 messenger RNA vaccination: a Japanese case series. Intern Med. 2022:8721–8731. doi: 10.2169/internalmedicine.8731-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naghashzadeh F., Shafaghi S., Dorudinia A., Naji S.A., Marjani M., Amin A., et al. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: case report. ESC Hear Fail. 2022;9:1483–1486. doi: 10.1002/ehf2.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee C.-H., Kong E.-J. FDG PET/MRI of acute myocarditis after mRNA COVID-19 vaccination. Clin Nucl Med. 2022;47:e421–e422. doi: 10.1097/RLU.0000000000004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fosch X., Serra J., Torres P.L., Preda L., González R., Mojer F. Acute myocarditis after a third dose of the BNT162b2 COVID-19 vaccine. Rev Española Cardiol (English. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes D.A., Santos R.R., Freitas P., Paiva M.S., Ferreira J., Trabulo M. Acute myocarditis following mRNA COVID-19 vaccine. Arq Bras Cardiol. 2022;118:787–788. doi: 10.36660/abc.20210469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brage Et, Ruíz J.R., Martín J.G., Rodríguez J.D.O., Tocino R.V., Rodríguez-Diego S., et al. Fulminant myocarditis IN a patient with a lung adenocarcinoma after the third dose OF modern COVID-19 vaccine. A case report and literature review. Curr Probl Cancer Case Reports. 2022;6 doi: 10.1016/j.cpccr.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharbatdaran A., Chahal Y., Molaei M., Bhavsar D. A rare case of COVID-19 vaccine-induced myopericarditis in a young adult. Radiol Case Reports. 2022;17:1916–1920. doi: 10.1016/j.radcr.2022.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moosmann J., Gentles T., Occleshaw C., Mitchelson B. COVID vaccine-associated myocarditis in adolescent siblings: does it run in the family? Vaccines. 2022;10:611. doi: 10.3390/vaccines10040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sciaccaluga C., D'Ascenzi F., Cameli M., Gallotta M., Menci D., Antonelli G., et al. Case report: two case reports of acute myopericarditis after mRNA COVID-19 vaccine. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.827237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agdamag A.C.C., Gonzalez D., Carlson K., Konety S., McDonald W.C., Martin C.M., et al. Fulminant myocarditis following coronavirus disease 2019 vaccination: a case report. Eur Hear Journal-Case Reports. 2022;6 doi: 10.1093/ehjcr/ytac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nunn S., Kersten J., Tadic M., Wolf A., Gonska B., Hüll E., et al. Case report: myocarditis after COVID-19 vaccination–case series and literature review. Front Med. 2022;9 doi: 10.3389/fmed.2022.836620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parmar K., Mekraksakit P., Del Rio-Pertuz G., Sethi P., Motes A., Hughes M., et al. vol. 35. Taylor & Francis; 2022. Myocarditis following COVID-19 mRNA vaccination; pp. 209–213. (Baylor univ med cent proc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dlewati M., Park K., Rawat S., Conte J., Bhadha K. COVID-19 mRNA vaccine-associated myocarditis presenting as STEMI in a 48-year-old male. Case Reports Cardiol. 2022:2022. doi: 10.1155/2022/2284530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kojima N., Tada H., Okada H., Yoshida S., Sakata K., Usui S., et al. Case report: myocarditis associated with COVID-19 mRNA vaccination following myocarditis associated with Campylobacter jejuni. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.837759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharff K.A., Dancoes D.M., Longueil J.L., Lewis P.F., Johnson E.S. Myopericarditis after COVID-19 booster dose vaccination. Am J Cardiol. 2022 doi: 10.1016/j.amjcard.2022.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chellapandian S.B., Turkmen S., Salim I., Chinnakaruppan S., Mohammad J. Myocarditis following COVID-19 mRNA (mRNA-1273) vaccination. Clin Case Reports. 2022;10 doi: 10.1002/ccr3.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.