Abstract
Rationale and Objective.
Achievement of decongestion in acute heart failure (AHF) is associated with improved survival and cardiovascular outcomes, but can be associated with acute declines in estimated glomerular filtration rate (eGFR). We sought to examine whether rate of in-hospital decongestion is associated with longer term kidney function decline.
Study Design.
Post hoc analysis of trial data.
Settings & Participants.
Patients with ≥2 measures of kidney function (n=3,500) from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial.
Exposure.
In-hospital rate of change in assessments of volume overload, including b-type natriuretic peptide (BNP), N-terminal pro b-type natriuretic peptide (NT-proBNP) and clinical congestion score (0–12); and rate of change in hemoconcentration including measures of hematocrit, albumin and total protein.
Outcomes.
Incident chronic kidney disease (CKD) Stage ≥4 (defined by a new eGFR<30 ml/min/1.73m2) and eGFR decline of >40%.
Analytical Approach.
Multivariable cause-specific hazards models.
Results.
Over median 10-month follow-up, faster decreases in volume overload and more rapid increases in hemoconcentration were associated with decreased risk of incident CKD Stage ≥4 and eGFR decline of >40%. In adjusted analyses, for every 6% faster decline in BNP per week, there was a 32% lower risk of both incident CKD Stage ≥4 (HR=0.68, 95% CI 0.58, 0.79) and eGFR decline by >40% (HR=0.68, 95% CI 0.57, 0.80). For every 1% faster increase per week in absolute hematocrit, there was a lower risk for both incident CKD Stage ≥4 (HR=0.73 [0.64, 0.84]) and eGFR decline by >40% (HR=0.82 [0.71, 0.95]), with results consistent for other biomarkers.
Limitations.
Possibility of residual confounding.
Conclusion.
These results provide reassurance that more rapid decongestion in patients with AHF do not increase the risk of adverse kidney outcomes in patients with HF.
Keywords: Cardiorenal syndrome, acute heart failure, decongestion, chronic kidney disease, hemoconcentration
PLAIN-LANGUAGE SUMMARY
For patients with heart failure, fluid accumulation in the lungs or other parts of the body causing shortness of breath or swelling usually prompt hospital admission. During the process of fluid removal, or decongestion, it is unknown whether the kidneys are placed at risk of worse longer term function if decongestion is allowed to occur faster. This study examined whether the rate of decongestion during hospitalizations for heart failure was associated with increased risk of worse kidney function in the 10-month period after discharge, using 3500 participants from the previously conducted EVEREST trial. The results show that more rapid decongestion did not increase the risk of worse longer term kidney function.
Introduction
Reduced kidney function is highly prevalent among patients with heart failure with reduced ejection fraction (HFrEF) and is associated with adverse clinical outcomes.1–4 Declines in estimated glomerular filtration rate (eGFR), occurring over many months, are also associated with increased risk of mortality and cardiovascular events.5 Prior studies have focused on risk factors for short-term declines in eGFR, such as those occurring during hospitalizations for acute heart failure (AHF), but few studies have examined risk factors for longitudinal declines in eGFR in HFrEF.
One hallmark of HFrEF and the most frequent reason for hospitalization for AHF is volume overload. The degree of volume overload has been shown to be an important risk factor for poor clinical outcomes, with greater degree of congestion at the time of admission for AHF being associated with higher risk of both poor cardiovascular outcomes,6,7 as well as with declines in kidney function.8,9 Evidence of successful decongestion, or fluid removal, by either improvement in clinical signs and symptoms or by evidence of hemoconcentration is associated with decreased risk of mortality.10 Because baseline congestion, as well as residual congestion at the time of hospital discharge,11,12 are both such powerful risk factors for poor clinical outcomes, the priority during hospitalizations for AHF usually revolves around decongestion. Some clinicians, however, grow concerned with rapid rates of decongestion and slow the rate of fluid removal out of concern for intravascular volume depletion and kidney injury. While there is a literature showing that decongestion can be associated with acute declines in eGFR that are not necessarily associated with worse clinical outcomes,13–15 what is less known is whether rate of decongestion is associated with longer term kidney function decline.
We hypothesized that faster rates of decongestion, by both improving cardiac function and decreasing renal venous pressure, would lead to improved long term kidney function among patients with HFrEF. Using data from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial,16 we evaluated whether the rate of improvement in two domains of assessments of congestion—1) volume overload, as assessed by fall in natriuretic peptides and improving clinical signs and symptoms, and 2) hemoconcentration as measured by increase in hematocrit, albumin and total protein—would be associated with longer term kidney outcomes.
Methods
Study Population and Design
The EVEREST trial was a multi-center randomized controlled trial that investigated the use of the vasopressin V2 receptor blocker tolvaptan in patients with HFrEF.16 Conducted from 2003 to 2006, it enrolled patients with reduced left ventricular ejection fraction (≤40%) who were admitted for AHF with evidence of congestion based upon ≥2 clinical signs or symptoms. Patients were randomized to receiving either 30mg of tolvaptan or placebo for a minimum of 60 days, in addition to standard medical therapy and were followed for a maximum of 2.5 years and median of 10 months. Key exclusion criteria included a serum creatinine >3.5 mg/dl and any comorbid condition with an expected survival of <6 months. Participants who died during hospitalization or had <2 measures of kidney function were excluded from the current analysis. Participants in EVEREST provided informed consent at the time of enrollment; the present study was deemed exempt from review by the Tufts Health Sciences Institutional Review Board.
Exposure
Change in Volume Overload
Volume overload was assessed both by biomarkers including BNP and NT-proBNP, and by clinical signs and symptoms. For administrative reasons, some centers collected BNP while others collected NT-proBNP; all samples were shipped and assayed at a central laboratory. Measurements were collected at the time of randomization and at pre-determined intervals during the hospitalization of Day 3, Day 7 and day of discharge (unless discharged sooner). Criteria for discharge were determined by the physician-investigators, who evaluated clinical signs and symptoms of volume overload at the time of randomization into the trial and then daily throughout the duration of the hospitalization. These included a standardized 4-point graded scale for pedal edema (absent/trace, slight, moderate, marked), JVD (≤6 cm, 6–9cm, 10–15cm, >15cm), rales (none, bases, up to <50%, to >50%) and orthopnea (none, seldom, frequent, continuous) which were then incorporated into a single congestion score with range from 0 to 12. While there is yet to be a validated and widely accepted standardized score for grading volume overload based on physical exam, this scale is a modification of a previously published congestion score based on data from the EVEREST trial6 and comprises the findings believed to be most specific to volume overload.17 For all three measures of volume overload, linear mixed models with a random intercept and slope were used to derive the in-hospital slope per week. Given the non-normal distribution of BNP and NT-proBNP, linear mixed models were fitted to log-transformed BNP and NT-proBNP, and then back transformed to the percent change for ease of interpretation.
Change in Hemoconcentration
Biomarkers of hemoconcentration included change in hematocrit, albumin, and total protein over the duration of the hospitalization. These biomarkers were measured at the time of randomization, and then at pre-determined intervals of Day 3, Day 7 and day of discharge (unless discharged sooner). Linear mixed models were used to derive the in-hospital slope in each biomarker per week.
Outcomes
The primary kidney function outcome of interest included incident CKD Stage ≥4 as defined by a new eGFR of ≤30 ml/min/1.73m2 given its association with adverse cardiac and kidney outcomes as well as predilection for development of complications from reduced kidney function.18 Our secondary outcome of interest was a decline of eGFR by >40% since it is accepted as a surrogate end-point in trials of CKD.19–21 Kidney function was estimated by using serum creatinine and the CKD-ERI formula.22 Given that our exposure variable was slope of decongestion during the hospitalization, our primary analyses started at time of discharge. Serum creatinine was measured at the time of hospital discharge and then every 4-weeks thereafter during follow-up. Less than 1% of measurements of creatinine were obtained outside of these pre-specified protocol visits, and as indication for testing was unclear, these were excluded from our analysis. Given the known variability in kidney function during in-hospital treatment for AHF and the fact that the relation of short-term declines in eGFR with outcomes is controversial,13,23,24 only kidney endpoints occurring after discharge from the initial hospitalization were included. In order to avoid short-term perturbation in hemodynamics that resulted in reaching the kidney outcomes, the kidney endpoint required two consecutive measures of eGFR. The exception was if the kidney outcome was based on the last measure of kidney function available, in which case a confirmatory measure was not required. Death and date of death (whether occurring before or after the kidney outcome) were also captured. Death prior to kidney outcome was a competing event.
Covariates
Several covariates were selected for analysis as potential confounding variables based on review of the literature and clinical relevance, including demographic characteristics (age, sex, race, body mass index [BMI]), severity of cardiac disease including ejection fraction, New York Heart Association functional class, and systolic blood pressure, medication (angiotensin-converting enzyme inhibitors [ACEI] or angiotensin II receptor blockers [ARB], mineralocorticoid receptor antagonists [MRA]), as well as randomization arm (tolvaptan or placebo).
Statistical Analysis
Continuous variables were summarized as mean ± SD for normally distributed variables, or median [25th, 75th percentiles] for non-normally distributed variables; categorical variables were summarized as frequency and percentages. Baseline characteristics were compared by quartiles of slope of decongestion using analysis of variance and Kruskal-Wallis tests, as well as by χ2 and Fisher’s exact tests for categorical and continuous variables.
The functional relation between each exposure and outcome was examined using restricted cubic splines, with 4 knots placed at the 5%, 35%, 65% and 95% percentiles of the distribution of the exposure variables. Given that there was no evidence of nonlinearity for all exposure variables, the linear slope was examined per standard deviation (SD) of slope for each exposure to allow for some uniformity in interpretations of rates of decongestion across the different domains of surrogate measures. Multivariable cause-specific hazard models were used to evaluate the association between the slope derived from linear mixed models of in-hospital change in each variable with each kidney outcome of interest. Time at risk for each kidney outcome began at the time of discharge. Patients were censored at the date of their last creatinine measurement. For the outcome of CKD Stage ≥4, patients who had an eGFR<30 ml/min/1.73m2 at the time of hospital discharge were excluded. In addition to providing cause-specific hazard ratios to summarize the association between slopes and kidney outcomes, we derived direct adjusted cumulative incidence curves of each kidney outcome according to quartiles of exposure variable using sub-distribution hazards models.25 In addition to the above covariates, models were adjusted for each respective baseline measure of congestion (i.e. baseline BNP for models examining BNP slope), given the association between greater baseline degree of congestion and worse kidney function outcomes.8,9 In fully adjusted models, the discharge eGFR was included as a covariate.
Several sensitivity analyses were performed. Given that more rapid decongestion may lead to a larger in-hospital eGFR decline as well as a higher incidence of CKD Stage ≥4 by the time of discharge (and therefore removal from the incident CKD Stage ≥4 analyses), we performed a sensitivity analysis using baseline eGFR as the starting point, recognizing that this analysis is partially limited in that the starting point for the outcome variable (change in kidney function) occurs before the ending time-point of the exposure variable (slope of decongestion). For these analyses, models were adjusted for baseline rather than discharge eGFR. Acknowledging that many clinicians use changes in weight to guide decongestion, analyses were repeated using slopes of weight, with the caveat that it is less specific to decongestion than our primary exposure variables. Finally, we assessed for effect modification by baseline degree of congestion by including an interaction term by baseline biomarker of decongestion.
All analyses were performed using SAS Enterprise Guide (Version 7.12, Cary, NC) and R language (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
There were 3500 patients with both discharge and at least one additional kidney function measurement available who were included into the analysis. Median follow-up was 10.1 [5.5, 16.4] months, with median of 8 [5, 11] measures of creatinine over the duration of follow-up.
Baseline Patient Characteristics
Baseline characteristics by quartiles of slope of BNP are presented in Table 1, with Quartile 1 having the least rapid decline and even some increase (least negative or positive slope) and Quartile 4 with the fastest decline in BNP (most negative slope). Baseline characteristics by quartiles of slope of hematocrit are presented in Supplemental Table S1. Overall, 71% had hypertension, 38% had diabetes and median eGFR at the time of discharge was 55 [41, 72] ml/min/1.73 m2. The majority of patients had declines in measures of volume overload and increases in measures of hemoconcentration, with distribution of rates in change available in Supplemental Figure S1. Those with the least rapid rates of decongestion (Quartile 1) tended to have evidence of greatest volume overload, with higher baseline BNP and NT-proBNP at randomization, and higher congestion score. They also tended to have lower hematocrit, albumin and total protein. There was no difference in discharge eGFR among those with more rapid or less rapid decline in BNP (Table 1) or hematocrit (Table S1). In those with more rapid decongestion (Quartile 4), there was a steeper decline in in-hospital eGFR and more cases of incident CKD Stage ≥4 (Table 1 and Table S1).
Table 1.
Baseline characteristics according to quartile of BNP slope per week during hospitalization.
| All (15.8% to −49.7%) | Quartile 1 (15.8 % to −19.6%) | Quartile 2 (−19.6 % to −22.4%) | Quartile 3 (−22.4 % to −25.1%) | Quartile 4 (−25.1 % to −49.7%) | p-value | |
|---|---|---|---|---|---|---|
| n=2357 | n=589 | n=590 | n=589 | n=589 | ||
| Age at discharge, years | 65.3 ± 11.4 | 64.7 ± 11.7 | 66.0 ± 11.5 | 66.6 ± 11.1 | 64.0 ± 11.1 | 0.2 |
| Female sex | 615 (26.1) | 152 (25.8) | 121 (20.5) | 154 (26.2) | 188 (31.9) | 0.003 |
| Black race | 143 (6.1) | 23 (3.9) | 49 (8.3) | 37 (6.3) | 34 (5.8) | 0.4 |
| Hypertension | 1676 (71.1) | 401 (68.1) | 397 (67.3) | 423 (71.8) | 455 (77.3) | <0.001 |
| Diabetes | 866 (36.7) | 216 (36.7) | 222 (37.6) | 218 (37.0) | 210 (35.7) | 0.7 |
| BMI, kg/m2 | 27.3 ± 5.3 | 26.2 ± 5.1 | 27.0 ± 5.2 | 27.6 ± 5.2 | 28.4 ± 5.6 | <0.001 |
| Ejection fraction | 28 ± 8 | 28 ± 8 | 26 ± 8 | 28 ± 8 | 31 ± 7 | <0.001 |
| Ischemic etiology of LV dysfunction | 1567 (67.3) | 430 (73.6) | 362 (62.2) | 400 (69.1) | 375 (64.4) | <0.001 |
| Discharge Systolic Blood Pressure, mmHg | 116 ± 16 | 115 ± 17 | 114 ± 16 | 116 ± 16 | 119 ± 15 | <0.001 |
| Congestion Score at randomization | 5.1 ± 2.1 | 5.4 ± 2.0 | 5.1 ± 2.0 | 4.9 ± 2.1 | 5.0 ± 2.0 | <0.001 |
| Congestion Score, change per week | −3.4 ± 1.5 | −2.7 ± 1.6 | −3.8 ± 1.4 | −3.7 ± 1.4 | −3.5 ± 1.5 | <0.001 |
| NYHA functional class | ||||||
| Class 1 or 2 | 10 (0.4) | 0 (0.0) | 3 (0.5) | 5 (0.9) | 2 (0.3) | 0.2 |
| Class 3 | 1428 (60.6) | 326 (55.4) | 375 (63.6) | 383 (65.0) | 344 (58.5) | |
| Class 4 | 917 (38.9) | 262 (44.6) | 212 (35.9) | 201 (34.1) | 242 (41.2) | |
| Current smoking | 289 (12.3) | 85 (14.5) | 67 (11.4) | 60 (10.2) | 77 (13.1) | 0.7 |
| Medications | ||||||
| ACEI or ARB | 2035 (86.3) | 513 (87.1) | 491 (83.2) | 502 (85.2) | 529 (89.8) | 0.1 |
| MRA | 1389 (58.9) | 381 (64.7) | 334 (56.6) | 325 (55.2) | 349 (59.3) | 0.05 |
| Laboratory Tests | ||||||
| eGFR at randomization, ml/min/1.73 m2 | 59.2 ± 21.5 | 56.9 ± 21.7 | 57 ± 21.4 | 58.5 ± 21.1 | 64.4 ± 21 | <0.001 |
| eGFR at discharge, ml/min/1.73 m2 | 57.6 ± 22 | 57.4 ± 22.4 | 57.8 ± 22.5 | 56 ± 20.7 | 59.2 ± 22.5 | 0.3 |
| eGFR slope, ml/min/1.73 m2 per week | −0.2 ± 0.5 | −0.1 ± 0.6 | −0.1 ± 0.5 | −0.2 ± 0.5 | −0.3 ± 0.6 | <0.001 |
| eGFR <30 ml/min/1.73 m2 at randomization | 207 (9.7) | 64 (11) | 59 (10.2) | 55 (9.5) | 29 (5) | <0.001 |
| eGFR <30 ml/min/1.73 m2 at discharge | 229 (9.7) | 54 (9.2) | 65 (11) | 62 (10.5) | 48 (8.2) | 0.5 |
| BNP at randomization, pg/ml | 646 (271, 1389) | 1038 (433, 2007) | 856 (461, 1556) | 458 (237, 930) | 381 (159, 987) | <0.001 |
| BNP change per week, % | −22 ± 6 | −15 ± 5 | −21 ± 1 | −24 ± 1 | −29 ± 4 | <0.001 |
| NT-proBNP at randomization, pg/ml | 4158 (1906, 7691) | 7244 (3942, 15059) | 5042 (2758, 11442) | 3045 (1778, 6145) | 2789 (1044, 5325) | <0.001 |
| NT-proBNP Change per week, % | −36 ± 12 | −21 ± 12 | −34 ± 6 | −41 ± 6 | −47 ± 9 | <0.001 |
| Hematocrit at randomization, % | 42.0 (38.0, 46.0) | 42.0 (38.0, 45.0) | 42.0 (38.0, 46.0) | 43.0 (38.0, 46.0) | 43.0 (40.0, 47.0) | <0.001 |
| Hematocrit change per week, % | 0.7 ± 1.0 | 0.4 ± 1.1 | 0.7 ± 0.9 | 0.8 ± 0.9 | 0.9 ± 1.1 | <0.001 |
| Albumin at randomization, g/dL | 3.8 (3.4, 4.1) | 3.7 (3.3, 4.0) | 3.7 (3.4, 4.0) | 3.9 (3.5, 4.2) | 3.9 (3.6, 4.2) | <0.001 |
| Albumin change per week, g/dL | 0.08 ± 0.11 | 0.07 ± 0.12 | 0.08 ± 0.10 | 0.08 ± 0.09 | 0.10 ± 0.11 | <0.001 |
| Total protein at randomization, g/dL | 7.1 (6.6, 7.6) | 7.0 (6.6, 7.6) | 7.0 (6.5, 7.5) | 7.2 (6.7, 7.6) | 7.2 (6.8, 7.7) | <0.001 |
| Total protein change per week, g/dL | 0.23 ± 0.16 | 0.20 ± 0.19 | 0.24 ± 0.14 | 0.24 ± 0.14 | 0.26 ± 0.17 | <0.001 |
| Randomization Group | ||||||
| Tolvaptan | 1166 (49.5) | 292 (49.6) | 276 (46.8) | 300 (50.9) | 298 (50.6) | 0.4 |
| Days in Hospital | 7 (3, 11) | 11 (7, 16) | 5 (2, 8) | 5 (2, 8) | 7 (5, 11) | <0.001 |
Values presented as n (%), mean ± standard deviation or median (25th, 75th percentiles).
BMI: body mass index; JVD: jugular venous distention; NYHA: New York Heart Association; ACEI:angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; MRA: mineralocorticoid receptor blocker, eGFR: estimated glomerular filtration rate; BNP: b-type natriuretic peptide; NT-proBNP: N-terminal pro b-type natriuretic peptide
Change in Volume Overload and Kidney Endpoints
There was a linear relation between greater rate of decrease in BNP, NT-proBNP, and congestion score with lower risk of incident CKD Stage ≥4 (Panel A, Figure 1). In both unadjusted and adjusted analyses, a more rapid decline in BNP was associated with lower hazard for incident CKD Stage ≥4 (unadjusted HR=0.69 [0.61, 0.79] per every SD [approximately 6%] decrease in BNP per week; adjusted HR=0.68 [0.58, 0.79]) (Table 2). In reference to the quartile with the slowest decline in BNP, quartiles with more rapid decline were associated with lower hazard of reaching incident CKD Stage ≥4 (Figure 2A). There was a similar pattern for NT-proBNP (Table 2, Figure 2A). In regards to congestion score, a more rapid decline was also associated with lower hazard for incident CKD Stage ≥4 (HR=0.82 [0.71, 0.94] per every SD [approximately 1 point] decrease per week), with the association growing stronger after adjustment for the baseline congestion score (Table 2).
Figure 1.

Association between change in markers of volume overload (A) and markers of hemoconcentration (B) with risk of incident CKD Stage ≥4. Restricted cubic splines plots show that more rapid rates of decline in BNP, NT-proBNP and clinical congestion score (rightward direction of each x-axis corresponds to more rapid decrease) from randomization to discharge are linearly associated with decreased risk of incident CKD Stage ≥4 in Panel A. Similarly, more rapid rates of increase in hematocrit, albumin and total protein (rightward direction of each x-axis corresponds to more rapid increase) are linearly associated with decreased risk of incident CKD Stage ≥4 in Panel B. Pglobal represents statistical testing of each exposure when modeled using the restricted cubic splines, with pre-specified threshold of 5% or less suggesting that there is a significant association. When this pglobal is statistically significant, the statistical testing of the non-linear association of each exposure and outcome was performed and represented by plinear. For the analyses in which the pglobal and plinear are both statistically significant, there exists a significant association with the outcome and this association is non-linear. Adjusted for age, sex, race, randomization group (tolvaptan vs placebo), BMI, medication use (ACEI or ARB, MRA), ejection fraction, New York Heart Association functional class, systolic blood pressure, eGFR at discharge and respective baseline biomarker level (i.e. baseline BNP for model examining BNP slope).
Abbreviations: BNP, b-type natriuretic peptide; NT-proBNP: N-terminal pro b-type natriuretic peptide; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease
Table 2.
Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% based on rates of change in measures of volume overload
| Marker of Volume Overload | Continuous | Quartile 1 Least rapid decongestion |
Quartile 2 | Quartile 3 | Quartile 4 Most rapid decongestion |
|
|---|---|---|---|---|---|---|
| CKD Stage ≥4 | ||||||
|
BNP Per 6% decrease per week |
N | 2128 | 535 | 525 | 527 | 541 |
| Event | 170 | 53 | 54 | 48 | 15 | |
| FU time (y) | 1429 | 302 | 347 | 387 | 394 | |
| Rate (100py) | 11.9 | 17.6 | 15.6 | 12.4 | 3.8 | |
| Unadjusted | 0.69 (0.61, 0.79) | 1.00 (1.00, 1.00) | 0.86 (0.59, 1.26) | 0.68 (0.46, 1.01) | 0.21 (0.12, 0.38) | |
| Adjusted | 0.68 (0.58, 0.79) | 1.00 (1.00, 1.00) | 0.91 (0.62, 1.35) | 0.68 (0.45, 1.03) | 0.25 (0.14, 0.45) | |
|
NT-proBNP Per 12% decrease per week |
N | 1049 | 232 | 261 | 273 | 283 |
| Event | 123 | 39 | 40 | 28 | 16 | |
| FU time (y) | 1250 | 221 | 283 | 350 | 396 | |
| Rate (100py) | 9.8 | 176 | 14.1 | 8.0 | 4.0 | |
| Unadjusted | 0.64 (0.55, 0.76) | 1.00 (1.00, 1.00) | 0.81 (0.52, 1.26) | 0.43 (0.26, 0.70) | 0.23 (0.13, 0.41) | |
| Adjusted | 0.71 (0.58, 0.88) | 1.00 (1.00, 1.00) | 0.89 (0.56, 1.41) | 0.62 (0.37, 1.06) | 0.35 (0.18, 0.68) | |
|
Congestion
Score Per 1 point decrease per week |
N | 3071 | 774 | 779 | 772 | 746 |
| Event | 282 | 72 | 56 | 80 | 74 | |
| FU time (y) | 2497 | 559 | 667 | 645 | 624 | |
| Rate (100py) | 11.3 | 12.9 | 8.4 | 12.4 | 11.9 | |
| Unadjusted | 0.99 (0.87, 1.12) | 1.00 (1.00, 1.00) | 0.65 (0.46, 0.92) | 0.95 (0.69, 1.31) | 0.89 (0.64, 1.24) | |
| Adjusted | 0.82 (0.71, 0.94) | 1.00 (1.00, 1.00) | 0.61 (0.43, 0.87) | 0.66 (0.47, 0.92) | 0.59 (0.41, 0.86) | |
| eGFR Decline by >40% | ||||||
|
BNP Per 6% decrease per week |
N | 2357 | 589 | 590 | 589 | 589 |
| Event | 133 | 42 | 41 | 33 | 17 | |
| FU time (y) | 1591 | 330 | 393 | 434 | 432 | |
| Rate (100py) | 8.4 | 12.7 | 10.4 | 7.6 | 3.9 | |
| Unadjusted | 0.69 (0.59, 0.80) | 1.00 (1.00, 1.00) | 0.75 (0.49, 1.16) | 0.53 (0.34, 0.85) | 0.29 (0.16, 0.50) | |
| Adjusted | 0.68 (0.57, 0.80) | 1.00 (1.00, 1.00) | 0.65 (0.42, 1.02) | 0.54 (0.34, 0.87) | 0.31 (0.17, 0.56) | |
|
NT-proBNP Per 12% decrease per week |
N | 1188 | 297 | 297 | 297 | 297 |
| Event | 135 | 46 | 34 | 34 | 21 | |
| FU time (y) | 1401 | 270 | 335 | 372 | 424 | |
| Rate (100py) | 9.6 | 17.0 | 10.2 | 9.1 | 5.0 | |
| Unadjusted | 0.67 (0.58, 0.78) | 1.00 (1.00, 1.00) | 0.58 (0.37, 0.90) | 0.47 (0.30, 0.74) | 0.26 (0.16, 0.44) | |
| Adjusted | 0.74 (0.63, 0.88) | 1.00 (1.00, 1.00) | 0.65 (0.41, 1.03) | 0.55 (0.34, 0.90) | 0.30 (0.17, 0.53) | |
|
Congestion
Score Per 1 point decrease per week |
N | 3412 | 853 | 853 | 853 | 853 |
| Event | 247 | 63 | 51 | 62 | 71 | |
| FU time (y) | 2787 | 627 | 728 | 721 | 710 | |
| Rate (100py) | 8.9 | 10.0 | 7.0 | 8.6 | 10.0 | |
| Unadjusted | 0.96 (0.84, 1.09) | 1.00 (1.00, 1.00) | 0.64 (0.45, 0.93) | 0.78 (0.55, 1.11) | 0.88 (0.62, 1.24) | |
| Adjusted | 0.81 (0.69, 0.94) | 1.00 (1.00, 1.00) | 0.61 (0.42, 0.88) | 0.60 (0.42, 0.87) | 0.58 (0.39, 0.86) | |
Cause-specific hazard modeling for slope of markers of volume overload during hospitalization for AHF. Hazard ratios are interpreted per each standard deviation of slope per week (i.e., for BNP, standard deviation of slope was 6% decrease per week), allowing for some uniformity across the different variables of rates of change in decongestion. BNP and NT-proBNP are transformed on the log scale, enabling hazard ratios to be interpreted per percent decrease per week. Hazard ratios for slope of congestion score (range 0 to 12, with higher score indicative of greater congestion) are per every 1 point decrease per week.
Adjusted: Adjusted for age, sex, race, randomization group (tolvaptan vs placebo), BMI, medication use (ACEI or ARB, MRA), ejection fraction, New York Heart Association functional class, systolic blood pressure, eGFR at discharge and respective baseline biomarker level.
Abbreviations: BNP, b-type natriuretic peptide; NT-proBNP: N-terminal pro b-type natriuretic peptide; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease, FU time (y), total follow-up time in years, 100py, 100 person-years
Figure 2.

Direct adjusted cumulative incidence curves of incident CKD Stage ≥4 according to quartiles of change in markers of volume overload (A), and markers of hemoconcentration (B) over the months of follow-up. Direct adjusted cumulative incidence curves of the kidney outcome were fitted using subdistribution hazards models accounting for death prior to kidney outcome as a competing event. In reference to Quartile 1 (least rapid rate of decongestion), quartiles with more rapid rates of decongestion had lower proportions of reaching incident CKD Stage ≥4. The number at risk is at the bottom of each plot, over time in terms of months of follow-up.
Abbreviations: eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; BNP, b-type natriuretic peptide; NT-proBNP, N-terminal pro b-type natriuretic peptide
Associations between slopes of decrease in BNP, NT-proBNP and congestion score with our secondary outcome, risk of eGFR decline by >40%, were similar. That is, a greater rate of decrease in BNP, NT-proBNP, and congestion score were associated with lower hazard of decline in eGFR by >40% (Figure 3A). In adjusted analyses a more rapid decline in BNP was associated with lower hazard of 40% decline (HR=0.68 [0.57, 0.80] per every 6% decrease in BNP per week). In reference to the quartile with slowest decline in BNP, quartiles with more rapid decline were associated with lower hazard of reaching eGFR decline by >40% (Figure 4A). There was a similar pattern for both NT-proBNP and congestion score (Table 2, Figure 4A).
Figure 3.

Association between change in markers of volume overload (A) and markers of hemoconcentration (B) with risk of eGFR decline by >40% after hospitalization for acute decompensated heart failure. Restricted cubic splines plots show that more rapid rates of decline in BNP, NT-proBNP and clinical congestion score (rightward direction of each x-axis corresponds to more rapid decrease) from randomization to discharge are linearly associated with decreased risk of >40% eGFR decline in Panel A. Similarly, more rapid rates of increase in hematocrit, albumin and total protein (rightward direction of each x-axis corresponds to more rapid increase) are linearly associated with decreased risk of >40% eGFR decline in Panel B. Pglobal represents statistical testing of each exposure when modeled using the restricted cubic splines, with pre-specified threshold of 5% or less suggesting that there is a significant association. When this pglobal is statistically significant, the statistical testing of the non-linear association of each exposure and outcome was performed and represented by plinear. For the analyses in which the pglobal and plinear are both statistically significant, there exists a significant association with the outcome and this association is non-linear. Adjusted for age, sex, race, randomization group (tolvaptan vs placebo), BMI, medication use (ACEI or ARB, MRA), ejection fraction, New York Heart Association functional class, systolic blood pressure, eGFR at discharge and respective baseline biomarker level (i.e. baseline BNP for model examining BNP slope).
Abbreviations: eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; BNP, b-type natriuretic peptide; NT-proBNP, N-terminal pro b-type natriuretic peptide
Figure 4.
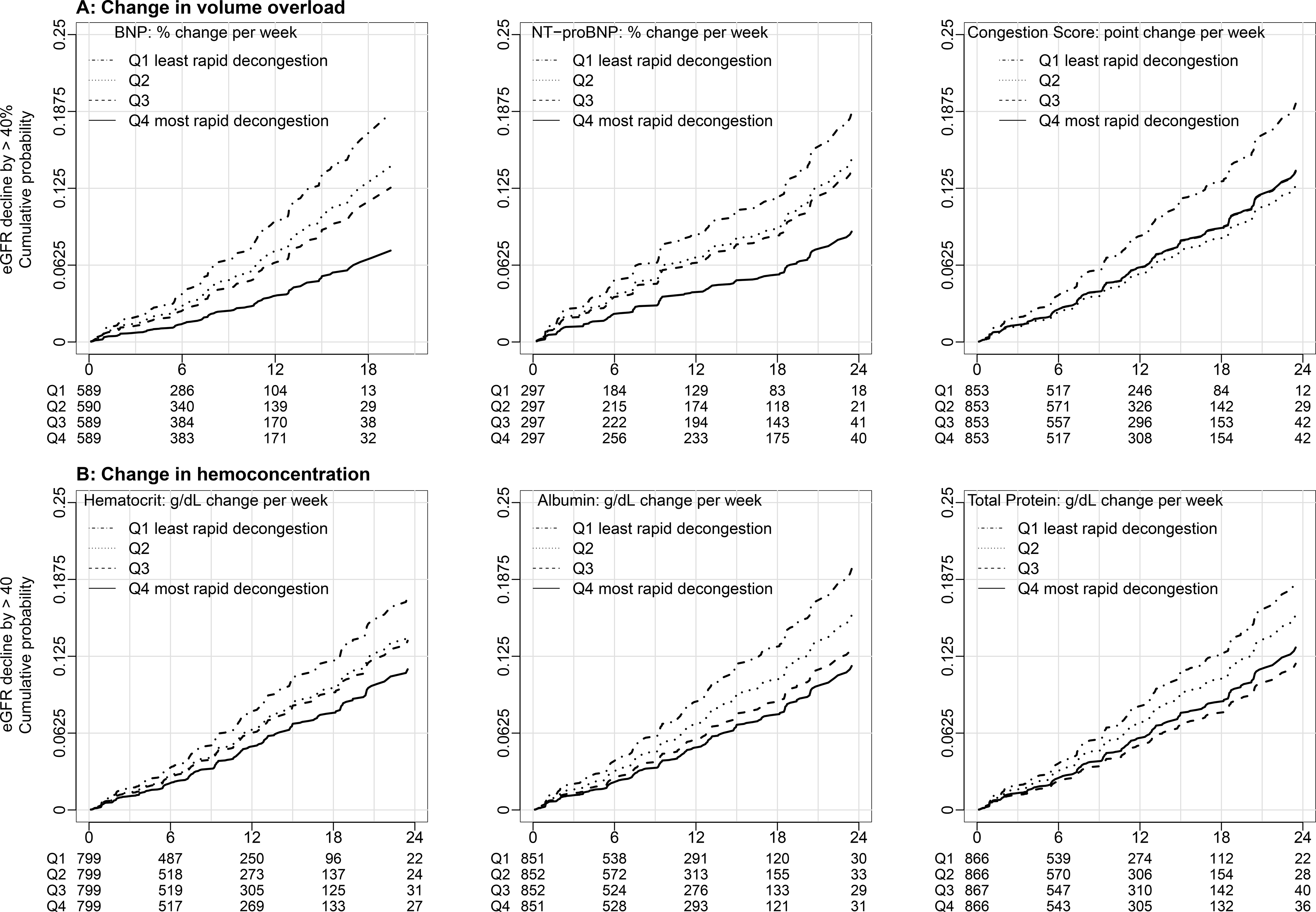
Direct adjusted cumulative incidence curves of eGFR decline by >40% according to quartiles of change in markers of volume overload (A) and markers of hemoconcentration (B). Direct adjusted cumulative incidence curves of the kidney outcome were fitted using subdistribution hazards models accounting for death prior to kidney outcome as a competing event. In reference to Quartile 1 (least rapid rate of decongestion), quartiles with more rapid rates of decongestion had lower proportions of reaching eGFR decline by >40%. The number at risk is at the bottom of each plot, over time in terms of months of follow-up. Abbreviations: eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; BNP, b-type natriuretic peptide; NT-proBNP, N-terminal pro b-type natriuretic peptide
Change in Hemoconcentration and Kidney Endpoints
There was a linear relation between greater rate of increase in hematocrit, albumin and total protein with lower risk of incident CKD Stage ≥4 (Panel B, Figure 1). In unadjusted analyses, there was a trend toward a more rapid increase in hematocrit being associated with lower hazard for incident CKD Stage ≥4 (Table 3), which grew stronger and reached statistical significance in adjusted analyses (HR=0.73 [0.64, 0.84] per every SD [approximately 1%] increase in hematocrit per week). In reference to the quartile with the slowest increase in hematocrit, quartiles with more rapid increase were associated with lower hazard of reaching incident CKD Stage ≥4 (Figure 2B). There was a similar pattern for both albumin and total protein (Table 3, Figure 2B). Associations between rates of increase in hematocrit and kidney outcomes became stronger after adjustment for baseline level of hematocrit; similar patterns were observed for albumin and total protein as well.
Table 3.
Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% based on rates of change in measures of hemoconcentration
| Measures of hemoconcentration | Continuous | Quartile 1 Least rapid decongestion |
Quartile 2 | Quartile 3 | Quartile 4 Most rapid decongestion |
|
|---|---|---|---|---|---|---|
| CKD Stage ≥4 | ||||||
|
Hematocrit Per 1% increase per week |
N | 2885 | 736 | 730 | 708 | 711 |
| Event | 250 | 60 | 60 | 83 | 47 | |
| FU time (y) | 2344 | 553 | 603 | 598 | 590 | |
| Rate (100py) | 10.7 | 10.9 | 9.9 | 13.9 | 8.0 | |
| Unadjusted | 0.90 (0.79, 1.02) | 1.00 (1.00, 1.00) | 0.92 (0.64, 1.31) | 1.27 (0.91, 1.77) | 0.73 (0.50, 1.07) | |
| Adjusted | 0.73 (0.64, 0.84) | 1.00 (1.00, 1.00) | 0.61 (0.43, 0.89) | 0.66 (0.46, 0.95) | 0.47 (0.31, 0.69) | |
|
Albumin Per 0.1 g/dL increase per week |
N | 3067 | 781 | 775 | 754 | 757 |
| Event | 274 | 63 | 66 | 79 | 66 | |
| FU time (y) | 2499 | 611 | 674 | 602 | 612 | |
| Rate (100py) | 11.0 | 10.3 | 9.8 | 13.1 | 10.8 | |
| Unadjusted | 1.00 (0.89, 1.12) | 1.00 (1.00, 1.00) | 0.95 (0.67, 1.34) | 1.27 (0.91, 1.77) | 1.05 (0.74, 1.48) | |
| Adjusted | 0.72 (0.63, 0.82) | 1.00 (1.00, 1.00) | 0.76 (0.53, 1.08) | 0.56 (0.39, 0.80) | 0.43 (0.30, 0.64) | |
|
Total
Protein Per 0.1 g/dL increase per week |
N | 3113 | 796 | 764 | 776 | 777 |
| Event | 281 | 63 | 61 | 81 | 76 | |
| FU time (y) | 2536 | 608 | 639 | 648 | 641 | |
| Rate (100py) | 11.1 | 10.4 | 9.6 | 12.5 | 11.9 | |
| Unadjusted | 1.00 (0.89, 1.13) | 1.00 (1.00, 1.00) | 0.92 (0.65, 1.31) | 1.20 (0.86, 1.67) | 1.14 (0.82, 1.60) | |
| Adjusted | 0.72 (0.62, 0.83) | 1.00 (1.00, 1.00) | 0.63 (0.43, 0.91) | 0.63 (0.43, 0.92) | 0.50 (0.34, 0.75) | |
| eGFR Decline by >40% | ||||||
|
Hematocrit Per 1% increase per week |
N | 3196 | 799 | 799 | 799 | 799 |
| Event | 227 | 54 | 52 | 71 | 50 | |
| FU time (y) | 2606 | 603 | 665 | 675 | 664 | |
| Rate (100py) | 8.7 | 9.0 | 7.8 | 10.5 | 7.5 | |
| Unadjusted | 0.96 (0.84, 1.09) | 1.00 (1.00, 1.00) | 0.83 (0.57, 1.22) | 1.11 (0.78, 1.58) | 0.81 (0.55, 1.20) | |
| Adjusted | 0.82 (0.71, 0.95) | 1.00 (1.00, 1.00) | 0.77 (0.53, 1.14) | 0.72 (0.49, 1.06) | 0.59 (0.40, 0.88) | |
|
Albumin Per 0.1 g/dL increase per week |
N | 3406 | 851 | 852 | 852 | 851 |
| Event | 245 | 63 | 62 | 61 | 59 | |
| FU time (y) | 2783 | 679 | 737 | 679 | 687 | |
| Rate (100py) | 8.8 | 9.3 | 8.4 | 9.0 | 8.6 | |
| Unadjusted | 1.00 (0.88, 1.13) | 1.00 (1.00, 1.00) | 0.88 (0.62, 1.25) | 0.95 (0.67, 1.36) | 0.93 (0.65, 1.32) | |
| Adjusted | 0.81 (0.71, 0.93) | 1.00 (1.00, 1.00) | 0.76 (0.53, 1.08) | 0.59 (0.40, 0.86) | 0.52 (0.35, 0.77) | |
|
Total
Protein Per 0.1 g/dL increase per week |
N | 3465 | 866 | 866 | 867 | 866 |
| Event | 251 | 61 | 64 | 59 | 67 | |
| FU time (y) | 2833 | 669 | 726 | 727 | 711 | |
| Rate (100py) | 8.9 | 9.1 | 8.8 | 8.1 | 9.4 | |
| Unadjusted | 0.97 (0.85, 1.10) | 1.00 (1.00, 1.00) | 0.94 (0.66, 1.34) | 0.84 (0.59, 1.21) | 1.00 (0.70, 1.41) | |
| Adjusted | 0.81 (0.70, 0.94) | 1.00 (1.00, 1.00) | 0.86 (0.60, 1.24) | 0.62 (0.41, 0.92) | 0.66 (0.44, 0.99) | |
Cause-specific hazard modeling for slope of markers of hemoconcentration during hospitalization for AHF. Hazard ratios are interpreted per each standard deviation of slope per week (i.e., for hematocrit, standard deviation of slope per week is 1% increase in hematocrit on absolute scale), allowing for some uniformity across the diferent variables of rates of change in decongestion.
Adjusted: Adjusted for age, sex, race, randomization group (tolvaptan vs placebo), BMI, medication use (ACEI or ARB, MRA), ejection fraction, New York Heart Association functional class, systolic blood pressure, eGFR at discharge and respective baseline biomarker level.
Abbreviations: BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease, FU time (y), total follow-up time in years, 100py, 100 person-years
Associations between slopes of hematocrit, albumin and total protein with our secondary kidney function outcome, eGFR decline by >40% were similar. That is, a greater rate of increase in hematocrit, albumin, and total protein were associated with lower hazard of decline in eGFR by > 40% (Figure 3B). In adjusted analyses a more rapid increase in hematocrit was associated with lower hazard of 40% decline (HR=0.82 [0.71, 0.95] per every 1% increase in hematocrit per week) (Table 3). In reference to the quartile with the slowest increase in hematocrit, quartiles with more rapid increase were associated with lower risk of reaching eGFR decline by >40% (Figure 4B). There was a similar pattern for both albumin and total protein (Table 3, Figure 4B).
Sensitivity Analysis
When designating the eGFR at the time of randomization (rather than discharge from hospitalization) as the starting point of each kidney outcome, associations between rates of decline in biomarkers of volume overload and incident CKD Stage ≥4 and eGFR decline >40% were slightly attenuated. With all three biomarkers of volume overload, a more rapid fall in BNP, NT-proBNP and congestion score was associated with a statistically significant decreased hazard of reaching eGFR decline by >40% (Table S2). For incident CKD Stage ≥4, the association was attenuated but remained statistically significant for BNP and NT-proBNP, and trended toward significance for congestion score (Table S2); in none of the analyses were there any trends toward increased risk. With all three biomarkers of hemoconcentration, a more rapid increase in hematocrit, albumin and total protein was also associated with decreased hazard of both kidney outcomes, albeit not meeting statistical significance for several of the analyses (Table S3). In none of the analyses were there any trends toward increased risk. When using in-hospital weight, there was no significant association between slope of weight with either kidney outcome (Table S4). We found no evidence of effect modification by baseline degree of congestion (Table S5); in other words, the association between slope of decongestion and outcomes was consistent regardless of the degree of initial congestion.
Discussion
This study indicates that more rapid achievement of decongestion (reduction in volume overload and increase in hemoconcentration) during hospitalization for AHF is associated with lower risk of incident CKD Stage ≥4 and eGFR decline by >40%. In none of the models, either unadjusted or adjusted, was more rapid achievement of decongestion associated with increased risk of either kidney outcome.
The classic paradigm of cardio-renal interactions in HFrEF has been that decreased perfusion from diminished cardiac output leads to reduced kidney function. However, a number of analyses in patients hospitalized with AHF have shown that neither baseline nor change in cardiac output had any association with change in kidney function.2,8,26 There has been evidence from animal models and observational studies that congestion, rather than cardiac output, are more strongly associated with declines in kidney function during hospitalizations for AHF.8,27,28 These findings are consistent with the literature evaluating mortality, which consistently show that indices of congestion and elevated cardiac filling pressures, rather than decreased cardiac output, are more important risk factors for poor clinical outcomes.29 The priority during treatment of AHF is therefore to decongest, with surrogate evidence of decongestion being achievement of hemoconcentration or decrease in natriuretic peptides, or clinically by improvement in edema or orthopnea. Much of the prior literature evaluating decongestion has examined it in the context of concomitant acute eGFR declines,13–15 suggesting that these acute declines in kidney function can be tolerated because decongestion improves cardiovascular outcomes and survival. However, clinicians often struggle with the question of how fast to allow decongestion to occur. While one observational study has suggested that faster rates of decongestion may be associated with mortality benefit,30 some clinicians tend to slow the rate of diuresis out of concern that large volumes of fluid loss will lead to intravascular volume depletion from inadequate plasma refill, risking the possibility of reduced renal perfusion and potentially causing kidney injury.31 However, there have not been any data indicating whether the rate of achieving decongestion is associated with clinically important longer term kidney function decline.
In this current analysis, we observed that more rapid decreases in volume overload (more rapidly falling BNP, NT-proBNP, congestion score) as well as more rapid increases in hemoconcentration (more rapidly rising hematocrit, albumin and total protein) were associated with lower risk of clinically important kidney function outcomes. Associations grew stronger particularly when adjusting for baseline of hemoconcentration, reflecting confounding from lower levels of hematocrit, albumin and total protein in those with most rapid hemoconcentration. Despite its wide use clinically, we did not observe any associations between changes in weight and outcomes, and others have shown that changes in weight are poorly correlated with measures of decongestion.32,33 There are two major interpretations to our results. The first is that rapid decongestion may be better for kidney-heart relations among patients with HFrEF, by leading to improvements in cardiac filling pressures and venous congestion, and thereby improved kidney function outcomes long term. This hypothesis is supported by studies showing that decongestion, even when there is concomitant evidence of renal tubular injury, has been associated with improved survival and short-term improvement in kidney function.34 The second is that rapid decongestion serves as a proxy for a healthier kidney with the ability to diurese rapidly, and therefore has better kidney function in follow-up. In either case, the rate of decongestion appears to be a valuable surrogate of longer term kidney outcomes, despite the observation that there is more acute eGFR decline among those with the fastest rates of decongestion. Even in the extremes of rapid decongestion within these current data, there is no evidence that more rapid decongestion is associated with adverse longer term kidney outcomes (Figures 1 and 3, Tables S2 and S3).
There are several limitations to consider. Participants of EVEREST did not have severe valvopathy or hemodynamic instability requiring cardiac mechanical support and thus these results may not apply to certain subgroups of patients with AHF. While adjusting for measures of severity of cardiac disease did not significantly alter associations, we cannot rule out the possibility of residual confounding. Kidney parameters such as albuminuria or dose titration of ACEIs/ARBs were not available. Even though our criteria for meeting each kidney outcome required a consecutive confirmatory measurement, there is a possibility that the kidney function outcomes reflect eGFR fluctuations known to occur in HFrEF, rather than progressive decline in kidney function. However, our kidney function outcomes relied on accepted definitions of surrogate endpoints, rather than minor fluctuations such as creatinine increase by 0.3 mg/dl. Furthermore, the use of a confirmatory second value of eGFR was used to eliminate risk of capturing random fluctuations in kidney function.
Among patients with HFrEF, more rapid rates of decongestion during hospitalizations for AHF were associated with decreased risk of future kidney function decline. That is, more rapid decline in BNP, NT-proBNP and clinical congestion score or more rapid increase in hematocrit, albumin or total protein were all associated with better long term kidney function. These results provide reassurance that more rapid rates of decongestion are not placing patients with HFrEF at risk for future kidney function decline.
Supplementary Material
Table S1: Baseline characteristics according to quartile of hematocrit slope per week during hospitalization
Table S2: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% starting from baseline eGFR based on rates of change in measures of volume overload
Table S3: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% starting from baseline eGFR based on rates of change in measures of hemoconcentration
Table S4: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% based on rates of change in weight
Table S5: Main effects of slopes of biomarkers of decongestion and interaction testing by baseline biomarker levels
Figure S1: Density plots of distribution of rates of change per week for each variable of volume overload or hemoconcentration
Support:
This work was supported by grants from the National Center for Advancing Translational Sciences (5 KL2 TR002545-04). The funders had no role in the study design, analysis, reporting, or decision to submit the manuscript for publication.
Financial Disclosure: JMT receives grant support from Otsuka and Abbott, as well as grants and consulting fees from BMS, 3ive labs, Boehringer Ingelheim, Bristol Myers Squibb, Reprieve Medical, FIRE1, Sanofi, Sequana Medical and Merck, and consulting fees from AstraZeneca, Novartis, Cardinomic, MagentaMed, W.I. Gore, Windtree Therapeutics, Lexicon pharmaceuticals, Regeneron, Edwards, BD, and Precardia. MJS sits on the Steering Committee for Akebia and is a consultant for Cardurian. JEU received grant support from Otsuka. MAK is the chair for the DSMB for BMS, and receives grant support from Otsuka and SC Pharma. The remaining authors declare that they have no relevant financial interests.
Footnotes
Peer Review: Received May 7, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form September 25, 2021. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572–580. doi: 10.1001/jama.293.5.572 [DOI] [PubMed] [Google Scholar]
- 2.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51(13):1268–1274. doi: 10.1016/j.jacc.2007.08.072 [DOI] [PubMed] [Google Scholar]
- 3.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38(4):955–962. doi: 10.1016/s0735-1097(01)01470-x [DOI] [PubMed] [Google Scholar]
- 4.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35(3):681–689. doi: 10.1016/s0735-1097(99)00608-7 [DOI] [PubMed] [Google Scholar]
- 5.Kiernan MS, Gregory D, Sarnak MJ, et al. Early and late effects of high- versus low-dose angiotensin receptor blockade on renal function and outcomes in patients with chronic heart failure. JACC Heart Fail. 2015;3(3):214–223. doi: 10.1016/j.jchf.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34(11):835–843. doi: 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423–433. doi: 10.1093/eurjhf/hfq045 [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCallum W, Tighiouart H, Testani JM, et al. Association of Volume Overload With Kidney Function Outcomes Among Patients With Heart Failure With Reduced Ejection Fraction. Kidney Int Rep. 2020;5(10):1661–1669. doi: 10.1016/j.ekir.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kociol RD, McNulty SE, Hernandez AF, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6(2):240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio-Gracia J, Demissei BG, Ter Maaten JM, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185–191. doi: 10.1016/j.ijcard.2018.01.067 [DOI] [PubMed] [Google Scholar]
- 12.Lala A, McNulty SE, Mentz RJ, et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8(4):741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–272. doi: 10.1161/CIRCULATIONAHA.109.933275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCallum W, Tighiouart H, Testani JM, et al. Acute Kidney Function Declines in the Context of Decongestion in Acute Decompensated Heart Failure. JACC Heart Fail. 2020;8(7):537–547. doi: 10.1016/j.jchf.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin M, Rao VS, Fleming J, et al. Effect on Survival of Concurrent Hemoconcentration and Increase in Creatinine During Treatment of Acute Decompensated Heart Failure. Am J Cardiol. 2019;124(11):1707–1711. doi: 10.1016/j.amjcard.2019.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstam MA, Gheorghiade M, Burnett JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 17.Thibodeau JT, Drazner MH. The Role of the Clinical Examination in Patients With Heart Failure. JACC Heart Fail. 2018;6(7):543–551. doi: 10.1016/j.jchf.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 18.Abboud H, Henrich WL. Clinical practice. Stage IV chronic kidney disease. N Engl J Med. 2010;362(1):56–65. doi: 10.1056/NEJMcp0906797 [DOI] [PubMed] [Google Scholar]
- 19.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–835. doi: 10.1053/j.ajkd.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Lambers Heerspink HJ, Mondal H, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64(6):848–859. doi: 10.1053/j.ajkd.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metra M, Cotter G, Senger S, et al. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circ Heart Fail. 2018;11(5):e004644. doi: 10.1161/CIRCHEARTFAILURE.117.004644 [DOI] [PubMed] [Google Scholar]
- 24.McCallum W, Tighiouart H, Kiernan MS, Huggins GS, Sarnak MJ. Relation of Kidney Function Decline and NT-proBNP With Risk of Mortality and Readmission in Acute Decompensated Heart Failure. Am J Med. Published online June 24, 2019. doi: 10.1016/j.amjmed.2019.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanberg JS, Sury K, Wilson FP, et al. Reduced Cardiac Index Is Not the Dominant Driver of Renal Dysfunction in Heart Failure. J Am Coll Cardiol. 2016;67(19):2199–2208. doi: 10.1016/j.jacc.2016.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72(1):49–61. doi: 10.1113/jphysiol.1931.sp002761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnett JC, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238(4):F279–282. doi: 10.1152/ajprenal.1980.238.4.F279 [DOI] [PubMed] [Google Scholar]
- 29.Cooper LB, Mentz RJ, Stevens SR, et al. Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J Card Fail. 2016;22(3):182–189. doi: 10.1016/j.cardfail.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oguri M, Ishii H, Takahara K, et al. Efficacy of Rapid Decongestion Strategy in Patients Hospitalized for Acute Heart Failure. Circ J. 2020;84(6):958–964. doi: 10.1253/circj.CJ-19-1128 [DOI] [PubMed] [Google Scholar]
- 31.Raichlin E, Haglund NA, Dumitru I, et al. Worsening renal function in patients with acute decompensated heart failure treated with ultrafiltration: predictors and outcomes. J Card Fail. 2014;20(5):376.e25–32. [PubMed] [Google Scholar]
- 32.Testani JM, Brisco MA, Kociol RD, et al. Substantial Discrepancy Between Fluid and Weight Loss During Acute Decompensated Heart Failure Treatment. Am J Med. 2015;128(7):776–783.e4. doi: 10.1016/j.amjmed.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail. 2014;2(3):298–305. doi: 10.1016/j.jchf.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 34.Rao VS, Ahmad T, Brisco-Bacik MA, et al. Renal Effects of Intensive Volume Removal in Heart Failure Patients With Preexisting Worsening Renal Function. Circ Heart Fail. 2019;12(6):e005552. doi: 10.1161/CIRCHEARTFAILURE.118.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline characteristics according to quartile of hematocrit slope per week during hospitalization
Table S2: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% starting from baseline eGFR based on rates of change in measures of volume overload
Table S3: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% starting from baseline eGFR based on rates of change in measures of hemoconcentration
Table S4: Hazard ratios for incident CKD Stage ≥4 and eGFR decline by >40% based on rates of change in weight
Table S5: Main effects of slopes of biomarkers of decongestion and interaction testing by baseline biomarker levels
Figure S1: Density plots of distribution of rates of change per week for each variable of volume overload or hemoconcentration


